PiplartineInducer of cell-death CAS# 20069-09-4 |
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20069-09-4 | SDF | Download SDF |
| PubChem ID | 637858 | Appearance | Powder |
| Formula | C17H19NO5 | M.Wt | 317.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | PPLGM,Piperlongumine | ||
| Solubility | DMSO : ≥ 100 mg/mL (315.12 mM) *"≥" means soluble, but saturation unknown. | ||
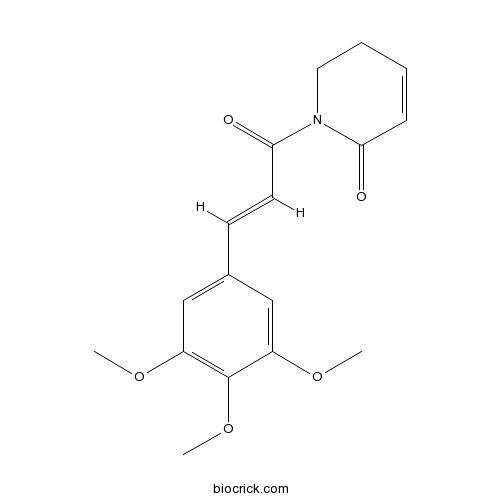
| Chemical Name | 1-[(E)-3-(3,4,5-trimethoxyphenyl)prop-2-enoyl]-2,3-dihydropyridin-6-one | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)C=CC(=O)N2CCC=CC2=O | ||
| Standard InChIKey | VABYUUZNAVQNPG-BQYQJAHWSA-N | ||
| Standard InChI | InChI=1S/C17H19NO5/c1-21-13-10-12(11-14(22-2)17(13)23-3)7-8-16(20)18-9-5-4-6-15(18)19/h4,6-8,10-11H,5,9H2,1-3H3/b8-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Piplartine shows potential anti-malaria and anti- leishmaniasis activities. 2. Piplartine shows inhibitory activities of platelet aggregation induced by ADP and AA in vitro . 3. Piplartine displays in vitro and in vivo anticancer efficacy. 4. Piplartine induces in vivo and in vitro mutagenicity in eukaryotic models. |
| Targets | P450 (e.g. CYP17) | Antifection |

Piplartine Dilution Calculator

Piplartine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1516 mL | 15.758 mL | 31.5159 mL | 63.0318 mL | 78.7898 mL |
| 5 mM | 0.6303 mL | 3.1516 mL | 6.3032 mL | 12.6064 mL | 15.758 mL |
| 10 mM | 0.3152 mL | 1.5758 mL | 3.1516 mL | 6.3032 mL | 7.879 mL |
| 50 mM | 0.063 mL | 0.3152 mL | 0.6303 mL | 1.2606 mL | 1.5758 mL |
| 100 mM | 0.0315 mL | 0.1576 mL | 0.3152 mL | 0.6303 mL | 0.7879 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Piperlongumine, a natural alkaloid from Piper longum L., increases the level of reactive oxygen species (ROS) and selectively kills cancer cells. It is a direct TrxR1 inhibitor with suppressive activity against gastric cancer and a novel inhibitor of CRM1; also an inhibitor of PI3K/Akt/mTOR in human breast cancer cells.
- Hennadiol
Catalog No.:BCN4679
CAS No.:20065-99-0
- Fmoc-D-Gln(Trt)-OH
Catalog No.:BCC3488
CAS No.:200623-62-7
- Fmoc-D-Gln-OPfp
Catalog No.:BCC3487
CAS No.:200622-33-9
- Fmoc-N-Me-Glu(OtBu)-OH
Catalog No.:BCC3213
CAS No.:200616-40-6
- Fmoc-D-Glu(OtBu)-OPfp
Catalog No.:BCC3497
CAS No.:200616-21-3
- Hotrienol
Catalog No.:BCN6340
CAS No.:20053-88-7
- GMX1778 (CHS828)
Catalog No.:BCC6527
CAS No.:200484-11-3
- Picralinal
Catalog No.:BCN4878
CAS No.:20045-06-1
- UB 165 fumarate
Catalog No.:BCC5746
CAS No.:200432-86-6
- 6-Chloroguanine riboside
Catalog No.:BCC8771
CAS No.:2004-07-1
- Fmoc-D-Cys(Trt)-OPfp
Catalog No.:BCC3482
CAS No.:200395-72-8
- Fmoc-D-Cit-OH
Catalog No.:BCC3181
CAS No.:200344-33-8
- (-)-Phyllocladene
Catalog No.:BCN7661
CAS No.:20070-61-5
- 7-Hydroxy-PIPAT maleate
Catalog No.:BCC6760
CAS No.:200722-46-9
- 16-Nor-15-oxodehydroabietic acid
Catalog No.:BCN3943
CAS No.:200813-31-6
- Pseudoneolinderane
Catalog No.:BCN8034
CAS No.:20082-45-5
- Epicurzerenone
Catalog No.:BCN3521
CAS No.:20085-85-2
- Diosbulbin B
Catalog No.:BCN4879
CAS No.:20086-06-0
- Diosbulbin C
Catalog No.:BCN4880
CAS No.:20086-07-1
- Epinodosin
Catalog No.:BCN3282
CAS No.:20086-60-6
- Xanthotoxol
Catalog No.:BCN4881
CAS No.:2009-24-7
- (D)-(+)-Neopterin
Catalog No.:BCC7960
CAS No.:2009-64-5
- m-3M3FBS
Catalog No.:BCC7209
CAS No.:200933-14-8
- SB 243213 dihydrochloride
Catalog No.:BCC6035
CAS No.:200940-23-4
Antitumour efficacy of Piper tuberculatum and piplartine based on the hollow fiber assay.[Pubmed:25519832]
Planta Med. 2015 Jan;81(1):15-9.
Piper tuberculatum, popularly known in Brazil as "jaborandi falso" and "pimenta darta", is widely used in folk medicine for the treatment of several diseases. In this study, the in vivo hollow fiber assay was used to investigate the antitumour efficacy of the crude extract and Piplartine obtained from P. tuberculatum roots. Human glioblastoma (SF-295) and colon carcinoma (HCT-8) cell lines were used. In vitro cytotoxicity was assayed by the MTT assay. In the hollow fiber assay, nude mice implanted with tumour cells in hollow fibers were treated for four consecutive days via the intraperitoneal route, and tumour cell populations were assessed by the MTT assay. Both the crude extract and Piplartine displayed cytotoxicity. In the hollow fiber assay, tumour growth inhibition rates were 24.6-54.8 % for the crude extract and 33.7-62.2 % for Piplartine. No signal of toxicity was noticed. In conclusion, the crude extract and Piplartine obtained from P. tuberculatum roots displayed in vitro and in vivo anticancer efficacy.
Effect of piplartine and cinnamides on Leishmania amazonensis, Plasmodium falciparum and on peritoneal cells of Swiss mice.[Pubmed:28415906]
Pharm Biol. 2017 Dec;55(1):1601-1607.
CONTEXT: Plants of the Piperaceae family produce Piplartine that was used to synthesize the cinnamides. OBJECTIVE: To assess the effects of Piplartine (1) and cinnamides (2-5) against the protozoa responsible for malaria and leishmaniasis, and peritoneal cells of Swiss mice. MATERIALS AND METHODS: Cultures of Leishmania amazonensis, Plasmodium falciparum-infected erythrocytes, and peritoneal cells were incubated, in triplicate, with different concentrations of the compounds (0 to 256 mug/mL). The inhibitory concentration (IC50) in L. amazonensis and cytotoxic concentration (CC50) in peritoneal cell were assessed by the MTT method after 6 h of incubation, while the IC50 for P. falciparum-infected erythrocytes was determined by optical microscopy after 48 or 72 h of incubation; the Selectivity Index (SI) was calculated by CC50/IC50. RESULTS: All compounds inhibited the growth of microorganisms, being more effective against P. falciparum after 72 h of incubation, especially for the compounds 1 (IC50 = 3.2 mug/mL) and 5 (IC50 = 6.6 mug/mL), than to L. amazonensis (compound 1 = 179.0 mug/mL; compound 5 = 106.0 mug/mL). Despite all compounds reducing the viability of peritoneal cells, the SI were <10 to L. amazonensis, whereas in the cultures of P. falciparum the SI >10 for the Piplartine (>37.4) and cinnamides 4 (>10.7) and 5 (= 38.4). DISCUSSION AND CONCLUSION: The potential of Piplartine and cinnamides 4 and 5 in the treatment of malaria suggest further pre-clinical studies to evaluate their effects in murine malaria and to determine their mechanisms in cells of the immune system.
Design, Synthesis and Pharmacological Evaluation of Novel Piperlongumine derivatives as Potential Antiplatelet Aggregation Candidate.[Pubmed:26706668]
Chem Biol Drug Des. 2016 Jun;87(6):833-40.
A series of novel piperlongumine derivatives (4a-i, 6a-i) were designed and synthesized. The inhibitory activities of platelet aggregation induced by ADP and AA in vitro have been evaluated by bron turbidimetry and liver microsomal incubated assay. The assay results show that compounds 4e and 6e exhibited remarkable potency to that of the positive control Piplartine and aspirin.
Piplartine induces genotoxicity in eukaryotic but not in prokaryotic model systems.[Pubmed:19379832]
Mutat Res. 2009 Jun-Jul;677(1-2):8-13.
Piplartine {5,6-dihydro-1-[(2E)-1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propen-1-yl]-2(1H)-pyridi none} is an alkamide present in Piper species that exhibits promising anticancer properties. It was previously shown that Piplartine is mutagenic in yeast and cultured mammalian cells. This study was performed to increase the knowledge on the mutagenic potential of Piplartine using the Salmonella/microsome assay, V79 cell micronucleus and chromosome aberration assays, and mouse bone-marrow micronucleus tests. Piplartine was isolated from the roots of Piper tuberculatum. This extracted compound was unable to induce a mutagenic response in any Salmonella typhimurium strain either in the presence or absence of metabolic activation. Piplartine showed mutagenic effects in V79 cells, as there was an increased frequency of aberrant cells and micronuclei formation. In addition, Piplartine administered at 50mg/kg did not induce micronucleus formation in vivo, but a dose of 100mg/kg induced an increase in the levels of micronucleus polychromatic erythrocytes (MNPCEs). Overall, these results provide further support that Piplartine induces in vivo and in vitro mutagenicity in eukaryotic models.
In vitro metabolism of the alkaloid piplartine by rat liver microsomes.[Pubmed:24667565]
J Pharm Biomed Anal. 2014 Jul;95:113-20.
Because Piplartine (PPT) has demonstrated biological activities, such as cytotoxic, anxiolytic, antidepressant, antifungal and antiplatelet activities, this molecule is a relevant drug candidate. The metabolic fate of drug candidates is an essential requirement in assessing their safety and efficacy. Based on this requirement, the biotransformation of PPT by cytochrome P450 enzymes (CYP) was investigated for the first time. To determine the in vitro enzymatic kinetic parameters, an HPLC method was developed and validated to quantify PPT. All samples were separated on a reversed-phase C18 column using a mobile phase of acetonitrile:water (40:60, v/v). The method exhibited a linear range of 2.4-157.7 mumol/L, with the following calibration curve: y=0.0934 (+/-0.0010)x+0.0027, r=0.9975. The lower limit of quantitation was verified to be 2.4 mumol/L, with an RSD below 7%. The precision and accuracy were assessed for both within-day and between-day determinations; neither relative standard (RSD%) deviations nor relative errors (RER) exceeded a value of 15%. The mean absolute recovery was 85%, with an RSD value below 6%. The enzymatic kinetic parameters revealed a sigmoidal profile, with V(max)=4.7+/-0.3 mumol/mg mL(-)(1)/min, h=2.5+/-0.4, S(5)(0)=44.7+/-0.3 mumol/L and CL(max)=0.054 muL/min/mg protein, indicating cooperativity behavior. Employing a mammalian model, PPT metabolism yielded two unreported monohydroxylated products (m/z 334). The identification and structural elucidation of the metabolites were performed by comparing their mass spectra with those spectra of the parent drug. For the first time, the in vitro metabolism studies employing microsomes were demonstrated to be a suitable tool for data regarding enzymatic kinetics and for the metabolites formed in the PPT mammalian metabolism.
Piperlongumine induces rapid depletion of the androgen receptor in human prostate cancer cells.[Pubmed:22592999]
Prostate. 2013 Jan;73(1):23-30.
BACKGROUND: Androgen receptor (AR) signaling is regarded as the driving force in prostate carcinogenesis, and its modulation represents a logical target for prostate cancer (PC) prevention and treatment. Natural products are the most consistent source of small molecules for drug development. In this study, we investigate the functional impact of piperlongumine (PL), a naturally occurring alkaloid present in the Long pepper (Piper longum), on AR expression in PC cells and delineate its mechanism of action. METHODS: Expression and transcriptional activity of AR was examined by western blotting and luciferase reporter assay, respectively. CellTiter Blue assay was utilized to quantify cell proliferation. Reactive oxygen species (ROS) generation was examined by staining cells with a ROS indicator CM-H(2) DCFDA, followed by flow cytometry analysis. RESULTS: The results of our experiments demonstrate that PL rapidly reduces AR protein levels in PC cells via proteasome-mediated ROS-dependent mechanism. Moreover, PL effectively depletes a modified AR lacking the ligand-binding domain, shedding light on a new paradigm in the treatment approach to prostatic carcinoma that expresses mutated constitutively active AR. Importantly, PL effectively depletes AR in PC cells at low micromolar concentrations, while concurrently exerting a significant inhibitory effect on AR transcriptional activity and proliferation of PC cells. CONCLUSIONS: Our investigation demonstrates for the first time that PL induces rapid depletion of the AR in PC cells. As such, PL may afford novel opportunities for both prevention and treatment of prostatic malignancy.
Selective killing of cancer cells by a small molecule targeting the stress response to ROS.[Pubmed:21753854]
Nature. 2011 Jul 13;475(7355):231-4.
Malignant transformation, driven by gain-of-function mutations in oncogenes and loss-of-function mutations in tumour suppressor genes, results in cell deregulation that is frequently associated with enhanced cellular stress (for example, oxidative, replicative, metabolic and proteotoxic stress, and DNA damage). Adaptation to this stress phenotype is required for cancer cells to survive, and consequently cancer cells may become dependent upon non-oncogenes that do not ordinarily perform such a vital function in normal cells. Thus, targeting these non-oncogene dependencies in the context of a transformed genotype may result in a synthetic lethal interaction and the selective death of cancer cells. Here we used a cell-based small-molecule screening and quantitative proteomics approach that resulted in the unbiased identification of a small molecule that selectively kills cancer cells but not normal cells. Piperlongumine increases the level of reactive oxygen species (ROS) and apoptotic cell death in both cancer cells and normal cells engineered to have a cancer genotype, irrespective of p53 status, but it has little effect on either rapidly or slowly dividing primary normal cells. Significant antitumour effects are observed in piperlongumine-treated mouse xenograft tumour models, with no apparent toxicity in normal mice. Moreover, piperlongumine potently inhibits the growth of spontaneously formed malignant breast tumours and their associated metastases in mice. Our results demonstrate the ability of a small molecule to induce apoptosis selectively in cells that have a cancer genotype, by targeting a non-oncogene co-dependency acquired through the expression of the cancer genotype in response to transformation-induced oxidative stress.


