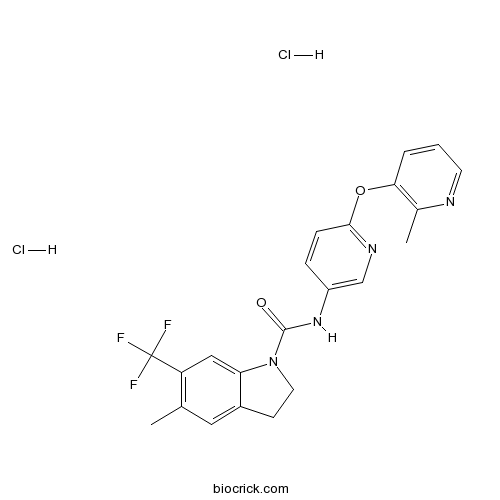
SB 243213 dihydrochlorideSelective 5-HT2C inverse agonist CAS# 200940-23-4 |
- Skepinone-L
Catalog No.:BCC1953
CAS No.:1221485-83-1
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 200940-23-4 | SDF | Download SDF |
| PubChem ID | 90488853 | Appearance | Powder |
| Formula | C22H21Cl2F3N4O2 | M.Wt | 501.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
| Chemical Name | 5-methyl-N-[6-(2-methylpyridin-3-yl)oxypyridin-3-yl]-6-(trifluoromethyl)-2,3-dihydroindole-1-carboxamide;dihydrochloride | ||
| SMILES | CC1=C(C=C2C(=C1)CCN2C(=O)NC3=CN=C(C=C3)OC4=C(N=CC=C4)C)C(F)(F)F.Cl.Cl | ||
| Standard InChIKey | UKMTYUXWLKQDNT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H19F3N4O2.2ClH/c1-13-10-15-7-9-29(18(15)11-17(13)22(23,24)25)21(30)28-16-5-6-20(27-12-16)31-19-4-3-8-26-14(19)2;;/h3-6,8,10-12H,7,9H2,1-2H3,(H,28,30);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective 5-HT2C inverse agonist (pKb = 9.8). Displays selectivity over other 5-HT2 subtypes (pKi values are 6.8, 7.0 and 9.0 for 5-HT2A, 5-HT2B and 5-HT2C respectively); also exhibits >100-fold selectivity over 50 other receptors, ion channels and enzymes. Displays anxiolytic activity in rat models. |

SB 243213 dihydrochloride Dilution Calculator

SB 243213 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9947 mL | 9.9735 mL | 19.9469 mL | 39.8939 mL | 49.8674 mL |
| 5 mM | 0.3989 mL | 1.9947 mL | 3.9894 mL | 7.9788 mL | 9.9735 mL |
| 10 mM | 0.1995 mL | 0.9973 mL | 1.9947 mL | 3.9894 mL | 4.9867 mL |
| 50 mM | 0.0399 mL | 0.1995 mL | 0.3989 mL | 0.7979 mL | 0.9973 mL |
| 100 mM | 0.0199 mL | 0.0997 mL | 0.1995 mL | 0.3989 mL | 0.4987 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- m-3M3FBS
Catalog No.:BCC7209
CAS No.:200933-14-8
- (D)-(+)-Neopterin
Catalog No.:BCC7960
CAS No.:2009-64-5
- Xanthotoxol
Catalog No.:BCN4881
CAS No.:2009-24-7
- Epinodosin
Catalog No.:BCN3282
CAS No.:20086-60-6
- Diosbulbin C
Catalog No.:BCN4880
CAS No.:20086-07-1
- Diosbulbin B
Catalog No.:BCN4879
CAS No.:20086-06-0
- Epicurzerenone
Catalog No.:BCN3521
CAS No.:20085-85-2
- Pseudoneolinderane
Catalog No.:BCN8034
CAS No.:20082-45-5
- 16-Nor-15-oxodehydroabietic acid
Catalog No.:BCN3943
CAS No.:200813-31-6
- 7-Hydroxy-PIPAT maleate
Catalog No.:BCC6760
CAS No.:200722-46-9
- (-)-Phyllocladene
Catalog No.:BCN7661
CAS No.:20070-61-5
- Piplartine
Catalog No.:BCN4037
CAS No.:20069-09-4
- Ac-RYYRWK-NH2
Catalog No.:BCC5755
CAS No.:200959-47-3
- Ac-RYYRIK-NH2
Catalog No.:BCC5736
CAS No.:200959-48-4
- Fmoc-Lys(Me)3-OH Chloride
Catalog No.:BCC3267
CAS No.:201004-29-7
- SB-269970
Catalog No.:BCC1927
CAS No.:201038-74-6
- Ravenine
Catalog No.:BCN6666
CAS No.:20105-22-0
- cis-Methylkhellactone
Catalog No.:BCN7690
CAS No.:20107-13-5
- 2-Amino-2'-chloro-5-nitro benzophenone
Catalog No.:BCC8521
CAS No.:2011-66-7
- 4-PPBP maleate
Catalog No.:BCC6723
CAS No.:201216-39-9
- 3-AQC
Catalog No.:BCC6743
CAS No.:201216-42-4
- Diosmetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN5328
CAS No.:20126-59-4
- H-Thr-OBzl.oxalate
Catalog No.:BCC3103
CAS No.:201274-07-9
- 1-(3,4-Dimethoxyphenyl)propane-1,2-diol
Catalog No.:BCN1507
CAS No.:20133-19-1
Differential effects of 5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxyl]-5-pyridyl]carbamoyl]-6-trifluoromethyli ndone (SB 243213) on 5-hydroxytryptamine(2C) receptor-mediated responses.[Pubmed:16807362]
J Pharmacol Exp Ther. 2006 Oct;319(1):260-8.
5-Methyl-1-[[2-[(2-methyl-3-pyridyl)oxyl]-5-pyridyl]carbamoyl]-6-trifluoromethyli ndone (SB 243213) is a selective, high-affinity 5-hydroxytryptamine (serotonin)(2C) receptor ligand that has been previously characterized as a competitive 5-HT(2C) receptor antagonist that has a long duration of activity in vivo. It is active in two preclinical models of anxiety and has an improved anxiolytic profile compared with benzodiazepines. In this study, we further characterized the pharmacological properties of SB 243213 by measuring its effects on each of multiple responses coupled to the 5-HT(2C) receptor. In Chinese hamster ovary cells, SB 243213 was an inverse agonist for the phospholipase A(2) response, for guanosine 5'-O-(3-[(35)S]thio)triphosphate binding, for reduction of constitutive desensitization, and for enhancement of dopamine release in the rat nucleus accumbens, with relative efficacies of 0.6, 1, 1, and 0.6, respectively. However, for the phospholipase C (PLC) signaling cascade, SB 243213 behaved as an antagonist. Although SB 243213 was previously characterized as a competitive antagonist for the PLC response, the magnitude of the dextral shift of the 5-HT concentration-response curve was time-dependent, and the maximal PLC response to 5-HT was decreased, probably as a result of the slow dissociation rate of SB 243213 (initial dissociation rate was 3.2 times slower than SB206553, a prototypical 5-HT(2C) receptor inverse agonist). Taken together, these data show that the pharmacological characteristics of SB 243213 at the 5-HT(2C) receptor differ depending upon the response measured, and they support the hypothesis that different drugs, acting at the same receptor subtype, can differentially regulate multiple cellular signaling systems.
SB-243213; a selective 5-HT2C receptor inverse agonist with improved anxiolytic profile: lack of tolerance and withdrawal anxiety.[Pubmed:11489455]
Neuropharmacology. 2001 Aug;41(2):186-99.
SB-243213 (5-methyl-1-[[-2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]-6-trifluoromethyl indoline hydrochloride) is a new, selective 5-hydroxytryptamine (5-HT)2C receptor inverse agonist. SB-243213 has high affinity for the human 5-HT2C receptor (pK(i) 9.37) and greater than a 100-fold selectivity over a wide range of neurotransmitter receptors, enzymes and ion channels. In in vitro functional studies, SB-243213 acted as an inverse agonist at the human 5-HT2C receptor with a pK(b) of 9.8. In in vivo studies, SB-243213 was a potent inhibitor of central 5-HT2C receptor-mediated function in rats, blocking meta-chlorophenylpiperazine-induced hypolocomotion with an ID50 of 1.1 mg/kg p.o. and a long duration of action (>8 h). In rats, SB-243213 exhibited anxiolytic-like activity in both the social interaction and Geller-Seifter conflict tests. Importantly, unlike diazepam, chronic administration of SB-243213 did not result in the development of either tolerance to the anxiolytic-like effects or withdrawal anxiogenesis. Furthermore, in rodents, SB-243213 did not affect seizure threshold, did not increase body weight or induce catalepsy, but attenuated the haloperidol-induced catalepsy. SB-243213 did not affect amphetamine-, MK-801- or phencyclidine-induced hyperactivity. In conclusion, SB-243213 may possess an improved anxiolytic profile compared to benzodiazepines. SB-243213 also modulates dopaminergic transmission, lacks pro-psychotic properties and may have utility in the treatment of schizophrenia and motor disorders.
Biarylcarbamoylindolines are novel and selective 5-HT(2C) receptor inverse agonists: identification of 5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]- 5-pyridyl]carbamoyl]-6-trifluoromethylindoline (SB-243213) as a potential antidepressant/anxiolytic agent.[Pubmed:10737744]
J Med Chem. 2000 Mar 23;43(6):1123-34.
The evolution, synthesis, and biological activity of a novel series of 5-HT(2C) receptor inverse agonists are reported. Biarylcarbamoylindolines have been identified with excellent 5-HT(2C) affinity and selectivity over 5-HT(2A) receptors. In addition, (pyridyloxypyridyl)carbamoylindolines have been discovered with additional selectivity over the closely related 5-HT(2B) receptor. Compounds from this series are inverse agonists at the human cloned 5-HT(2C) receptor, completely abolishing basal activity in a functional assay. The new series have reduced P450 inhibitory liability compared to a previously described series of 1-(3-pyridylcarbamoyl)indolines (Bromidge et al. J. Med. Chem. 1998, 41, 1598) from which they evolved. Compounds from this series showed excellent oral activity in a rat mCPP hypolocomotion model and in animal models of anxiety. On the basis of their favorable biological profile, 32 (SB-228357) and 40 (SB-243213) have been selected for further evaluation to determine their therapeutic potential for the treatment of CNS disorders such as depression and anxiety.


