Diosbulbin CCAS# 20086-07-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20086-07-1 | SDF | Download SDF |
| PubChem ID | 177108 | Appearance | Powder |
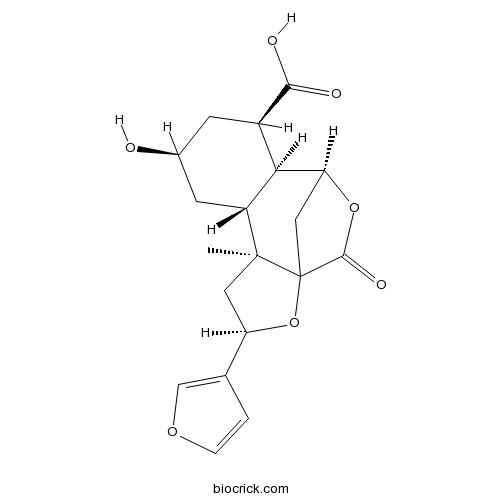
| Formula | C19H22O7 | M.Wt | 362.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC12CC(OC13CC(C4C2CC(CC4C(=O)O)O)OC3=O)C5=COC=C5 | ||
| Standard InChIKey | UYALWPKCIMKALF-XBHMPIGQSA-N | ||
| Standard InChI | InChI=1S/C19H22O7/c1-18-6-13(9-2-3-24-8-9)26-19(18)7-14(25-17(19)23)15-11(16(21)22)4-10(20)5-12(15)18/h2-3,8,10-15,20H,4-7H2,1H3,(H,21,22)/t10-,11+,12+,13+,14-,15+,18-,19?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Diosbulbin C has hepatotoxicity. |
| In vitro | An integrated metabolomics and proteomics approach to identify metabolic abnormalities in rats with Dioscorea bulbifera rhizome-induced hepatotoxicity.[Reference: WebLink]Chemical Research in Toxicology, 2018,31(9):843-851.It is vital to monitor the holistic toxicokinetics of toxic Chinese herbal medicines (CHMs) for safety. Although an integrated strategy based on the area under the curve (AUC) has been proposed to characterize the pharmacokinetic/toxicokinetic properties of CHMs, improvement is still needed. |

Diosbulbin C Dilution Calculator

Diosbulbin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7594 mL | 13.7969 mL | 27.5938 mL | 55.1876 mL | 68.9845 mL |
| 5 mM | 0.5519 mL | 2.7594 mL | 5.5188 mL | 11.0375 mL | 13.7969 mL |
| 10 mM | 0.2759 mL | 1.3797 mL | 2.7594 mL | 5.5188 mL | 6.8985 mL |
| 50 mM | 0.0552 mL | 0.2759 mL | 0.5519 mL | 1.1038 mL | 1.3797 mL |
| 100 mM | 0.0276 mL | 0.138 mL | 0.2759 mL | 0.5519 mL | 0.6898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Diosbulbin B
Catalog No.:BCN4879
CAS No.:20086-06-0
- Epicurzerenone
Catalog No.:BCN3521
CAS No.:20085-85-2
- Pseudoneolinderane
Catalog No.:BCN8034
CAS No.:20082-45-5
- 16-Nor-15-oxodehydroabietic acid
Catalog No.:BCN3943
CAS No.:200813-31-6
- 7-Hydroxy-PIPAT maleate
Catalog No.:BCC6760
CAS No.:200722-46-9
- (-)-Phyllocladene
Catalog No.:BCN7661
CAS No.:20070-61-5
- Piplartine
Catalog No.:BCN4037
CAS No.:20069-09-4
- Hennadiol
Catalog No.:BCN4679
CAS No.:20065-99-0
- Fmoc-D-Gln(Trt)-OH
Catalog No.:BCC3488
CAS No.:200623-62-7
- Fmoc-D-Gln-OPfp
Catalog No.:BCC3487
CAS No.:200622-33-9
- Fmoc-N-Me-Glu(OtBu)-OH
Catalog No.:BCC3213
CAS No.:200616-40-6
- Fmoc-D-Glu(OtBu)-OPfp
Catalog No.:BCC3497
CAS No.:200616-21-3
- Epinodosin
Catalog No.:BCN3282
CAS No.:20086-60-6
- Xanthotoxol
Catalog No.:BCN4881
CAS No.:2009-24-7
- (D)-(+)-Neopterin
Catalog No.:BCC7960
CAS No.:2009-64-5
- m-3M3FBS
Catalog No.:BCC7209
CAS No.:200933-14-8
- SB 243213 dihydrochloride
Catalog No.:BCC6035
CAS No.:200940-23-4
- Ac-RYYRWK-NH2
Catalog No.:BCC5755
CAS No.:200959-47-3
- Ac-RYYRIK-NH2
Catalog No.:BCC5736
CAS No.:200959-48-4
- Fmoc-Lys(Me)3-OH Chloride
Catalog No.:BCC3267
CAS No.:201004-29-7
- SB-269970
Catalog No.:BCC1927
CAS No.:201038-74-6
- Ravenine
Catalog No.:BCN6666
CAS No.:20105-22-0
- cis-Methylkhellactone
Catalog No.:BCN7690
CAS No.:20107-13-5
- 2-Amino-2'-chloro-5-nitro benzophenone
Catalog No.:BCC8521
CAS No.:2011-66-7
Describing the holistic toxicokinetics of hepatotoxic Chinese herbal medicines by a novel integrated strategy: Dioscorea bulbifera rhizome as a case study.[Pubmed:28910661]
J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Oct 1;1064:40-48.
It is vital to monitor the holistic toxicokinetics of toxic Chinese herbal medicines (CHMs) for safety. Although an integrated strategy based on the area under the curve (AUC) has been proposed to characterize the pharmacokinetic/toxicokinetic properties of CHMs, improvement is still needed. This study attempted to use 50% inhibitory concentration (IC50) as weighting coefficient to investigate holistic toxicokinetics of the major diosbulbins i.e. diosbulbin A (DA), diosbulbin B (DB), and Diosbulbin C (DC) after oral administration of Dioscorea bulbifera rhizome (DBR) extract. Firstly, the cytotoxicities of the three diosbulbins on human hepatic L02 cells were evaluated and the IC50 values were calculated. Then, integrated toxicokinetics of multiple diosbulbins based on AUC and IC50 were determined. Finally, correlations between integrated plasma concentrations and hepatic injury biomarkers including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bile acid (TBA) were analyzed. As a result, integrated plasma concentrations were correlated well with TBA and the correlation between TBA and IC50-weighting integrated plasma concentrations was better than that of AUC-weighting integrated plasma concentrations. In conclusion, the newly developed IC50-weighting method is expected to generate more reasonable integrated toxicokinetic parameters, which will help to guide the safe usage of DBR in clinical settings.


