Cyperus rotundus
Cyperus rotundus
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
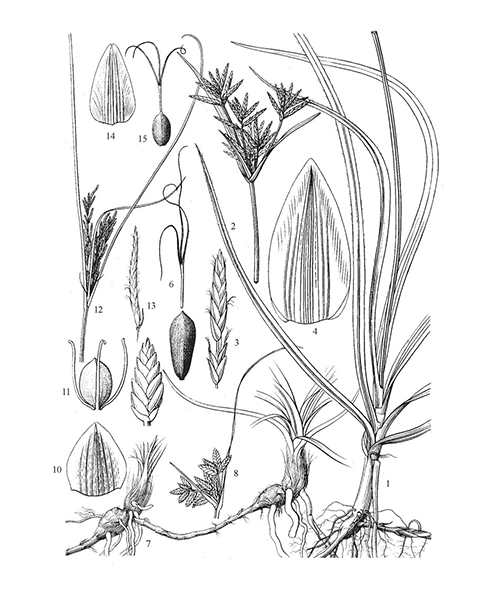
Natural products/compounds from Cyperus rotundus
- Cat.No. Product Name CAS Number COA
-
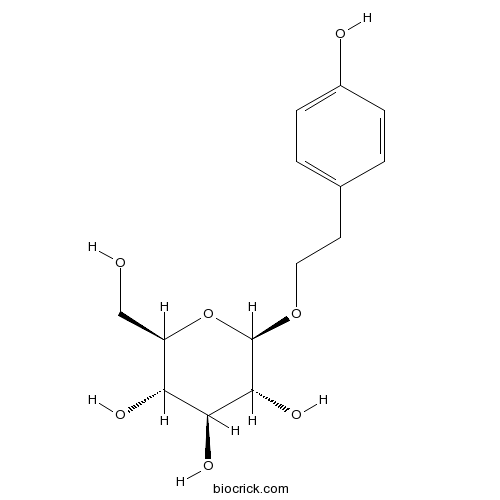
BCN5966
Salidroside10338-51-9
Instructions
-
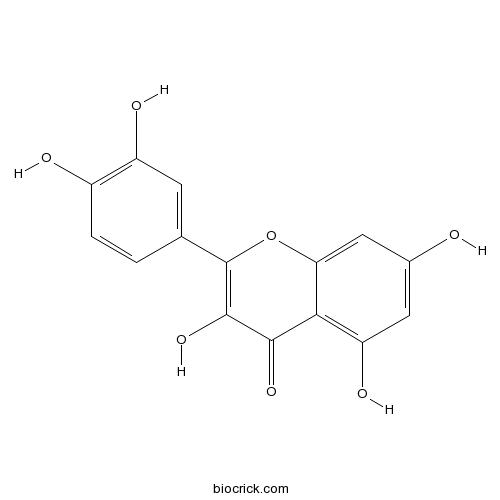
BCN6049
Quercetin117-39-5
Instructions
-
BCN3797
Limonene138-86-3
Instructions

-
BCN3857
(-)-beta-Pinene18172-67-3
Instructions

-
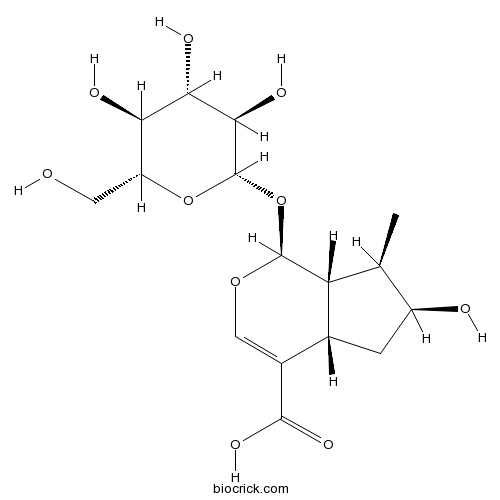
BCN5057
Loganic acid22255-40-9
Instructions
-
BCN5949
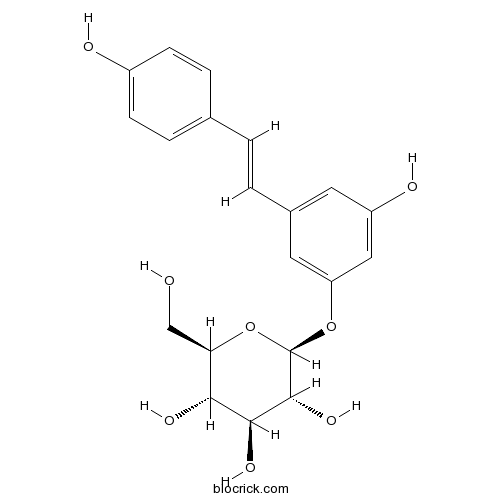
Polydatin27208-80-6
Instructions
-
BCN5979
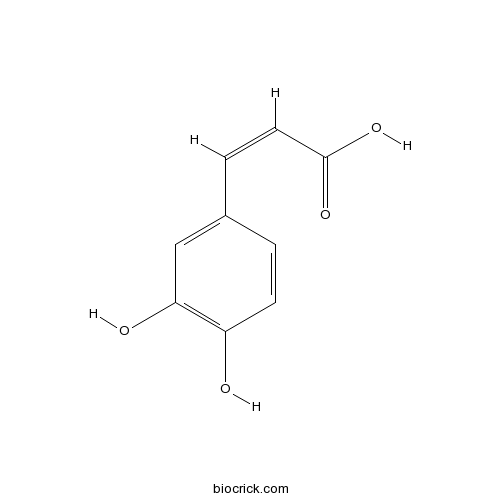
Caffeic acid331-39-5
Instructions
-
BCN8339
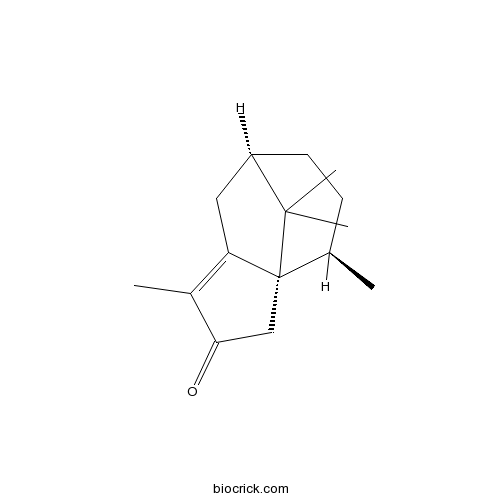
Cyperotundone3466-15-7
Instructions
-
BCN1193
alpha-Cyperone473-08-5
Instructions

-
BCN5536
Dehydrocostus lactone477-43-0
Instructions

-
BCN2319
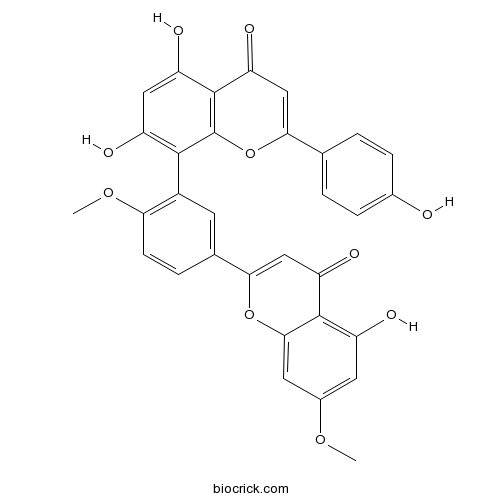
Ginkgetin481-46-9
Instructions
-
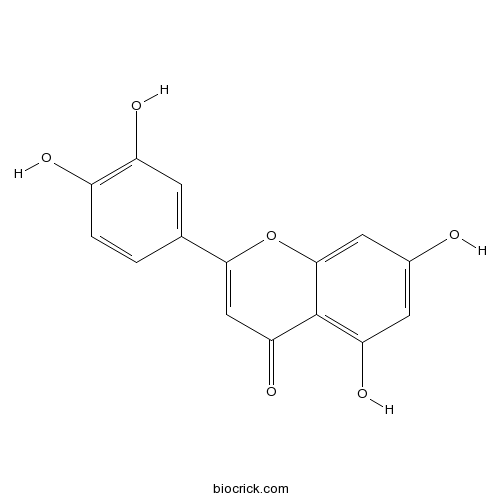
BCN5600
Luteolin491-70-3
Instructions
-
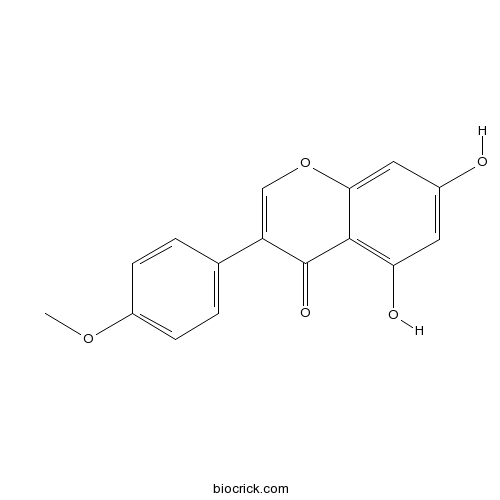
BCN1224
Biochanin A491-80-5
Instructions
-
BCN5616
Oleanolic acid508-02-1
Instructions

-
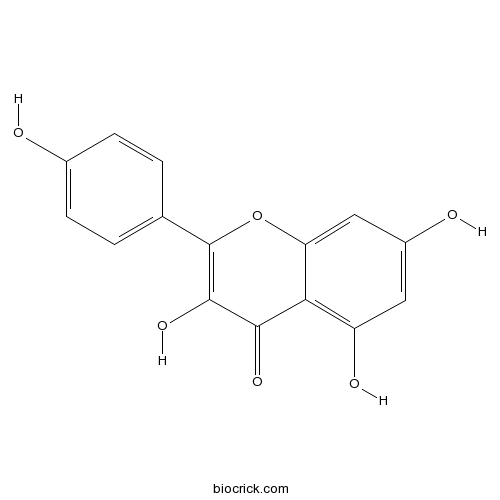
BCN5653
Kaempferol520-18-3
Instructions
-
BCN5663
Physcion521-61-9
Instructions

-
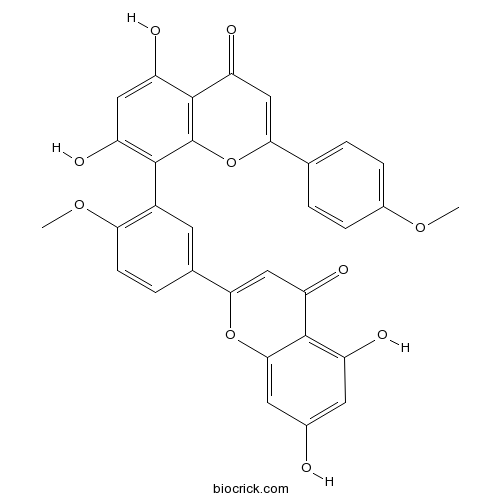
BCN2320
Isoginkgetin548-19-6
Instructions
-
BCC4109
Salicylic acid69-72-7
Instructions

-
BCN1015
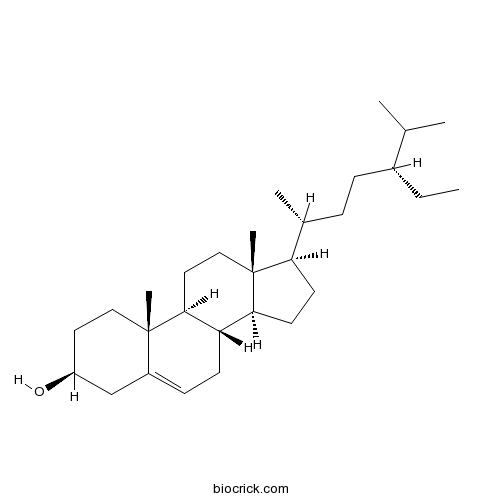
Beta-Sitosterol83-46-5
Instructions
-
BCN4376
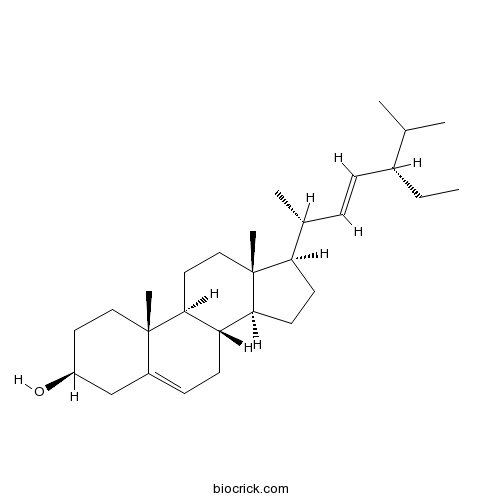
Stigmasterol83-48-7
Instructions
-
BCN6309
Coumarin91-64-5
Instructions

-
BCN4537
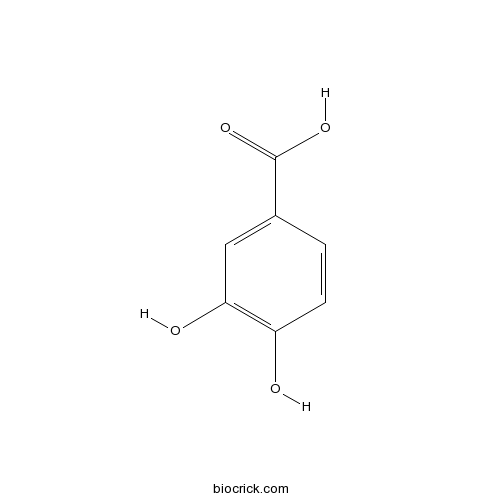
3,4-Dihydroxybenzoic acid99-50-3
Instructions
-
BCC8282
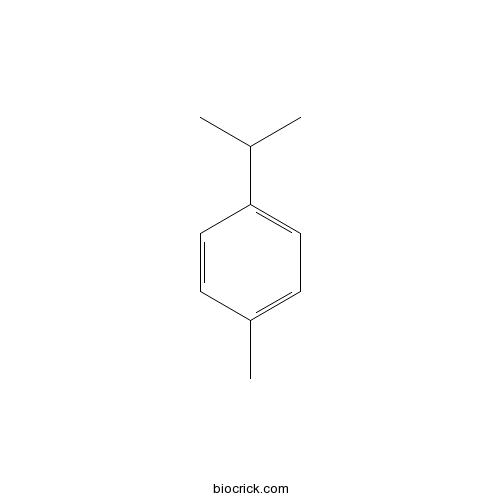
4-Isopropyltoluene99-87-6
Instructions
Effect of ethanolic extract of Cyperus rotundus L. against isoprenaline induced cardiotoxicity.[Pubmed: 30084568]
Interruption of blood supply to the heart results in acute myocardial infarction (AMI), and further damages the heart muscles. Available drugs for the treatment MI have one or other side effects, and there is a need for development of better alternative drugs from herbal sources. Here, we evaluated cardioprotective effect of Cyperus rotundus on isoprenaline- induced myocardial infarction. Thirty five Wistar rats, aged 60-100 days with body wt. 150-200 g, pretreated with ethanolic extract of Cyperus rotundus L. (@ 250 and 500 mg/kg body wt.) orally before induction of myocardial necrosis by administrating isoprenaline (85 mg/kg, s.c.) on 19th and 20th day of the pretreatment period. The treated rats were examined for gross functioning of heart, heart weight/body wt. Ratio, and also observed histopathologically. Further, activities of various cardiac enzymes such as aspartate transaminase, alanine transaminase, creatinine kinase-myoglobulin, lactate dehydrogenase, and the gold marker troponin-I were also determined. The levels altered by isoproterenol were found to be restored significantly by the test extracts especially at higher dose. Biochemical observations viz., serum ALT (P <0.0001), AST (P <0.0001), creatine kinase-myoglobulin (CK-MB) (P <0.0001), LDH (P <0.0001) demonstrated significant cardioprotective activity of the ethanolic extract of C. rotundus (500 mg/kg body wt.), against isoprenaline induced myocardial infarction. These results were also substantiated by physical parameters and histopathological observations. All these results were comparable with that of two standard drugs metoprolol (10 mg/kg/day), ramipril (3 mg/kg/day) as well as polyherbal formulation Abana (50 mg/kg/day).
Anti-inflammatory terpenoids from Cyperus rotundus rhizomes.[Pubmed: 30058534]
None
Synergy in the adulticidal efficacy of essential oils for the improvement of permethrin toxicity against Aedes aegypti L. (Diptera: Culicidae).[Pubmed: 30005688]
In a previous screening program for mosquitocides from local edible plants in Thailand, essential oils (EOs) of Cyperus rotundus, Alpinia galanga and Cinnamomum verum, were found to possess promising adulticidal activity against Aedes aegypti. With the aim of reducing usage of conventional insecticides and improving the management of resistant mosquito populations, this study was designed to determine the potential synergism in the adulticidal efficacy of EOs on permethrin toxicity against Ae. aegypti, both pyrethroid-resistant and -susceptible strains.
α-Cyperone inhibits LPS-induced inflammation in BV-2 cells through activation of Akt/Nrf2/HO-1 and suppression of the NF-κB pathway.[Pubmed: 29667667]
Accumulating evidence has shown that activated microglia cause inflammatory immune response, which could lead to neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease. α-Cyperone, one of the main ingredients of Cyperus rotundus oil, has been reported to possess anti-inflammatory activity in activated macrophages. In this study, we found that α-cyperone markedly decreased the production of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in LPS-induced BV-2 cells. Moreover, α-cyperone inhibited NF-κB activation and enhanced heme oxygenase-1 (HO-1), nuclear factor-E2-related factor 2 (Nrf2) and Akt expression. Furthermore, we found that α-cyperone could upregulate HO-1 expression and enhance nuclear translocation of Nrf2 via activating the Akt signaling pathway, and inhibition of Akt, Nrf2 or HO-1 attenuated LPS-induced expression of proinflammatory cytokines in BV-2 cells. Moreover, the toxicities of conditioned medium from activated microglia toward dopaminergic neuronal SH-SY5Y cells and hippocampal neuronal HT22 cells were significantly inhibited by pretreatment with α-cyperone. Taken together, our results indicate that α-cyperone exerts neuroprotective effects by inhibiting the production of inflammatory cytokines in BV-2 cells through activating Akt/Nrf2/HO-1 and suppressing the NF-κB pathway.
Multi-analytical strategy for unassigned peaks using physical/mathematical separation, fragmental rules and retention index prediction: An example of sesquiterpene metabolites characterization in Cyperus rotundus.[Pubmed: 29621725]
Comprehensive two-dimensional gas chromatography- mass spectrometry (GC × GC-qMS) can provide powerful physical separation, signal enhancement, and spectral identification for analytes in complex samples. Unassigned peaks are commonly presented in the untargeted profile after a single run with EI-MS spectral matching and retention index (RI) confirmation. The procedure proposed in this work can be applied as a general method for suggesting or narrowing down the candidates of unassigned GC × GC-qMS peaks. To begin, peak purity detection and chemometric resolution are employed to acquire pure mass spectra. In addition, the fragmental rules and in-silico spectra from structures are available for annotating certain unassigned peaks with reference spectra that are not observed in commercial databases. Furthermore, the procedure proposed in this work allows for in silico RI calculation by means of random forest (RF) analysis based on the retention data under the same chromatographic conditions. The calculated RIs can aid in analysis when the RI information of peaks of interest is not available in retention data libraries. Using the proposed strategy, certain unassigned peaks can be attributed to sesquiterpene metabolites in an in-house database for Cyperus rotundus.
A modified multiscale peak alignment method combined with trilinear decomposition to study the volatile/heat-labile components in Ligusticum chuanxiong Hort - Cyperus rotundus rhizomes by HS-SPME-GC/MS.[Pubmed: 29438837]
Head Space/Solid Phase Micro-Extraction (HS-SPME) coupled with Gas Chromatography/Mass Spectrometer (GC/MS) was used to determine the volatile/heat-labile components in Ligusticum chuanxiong Hort - Cyperus rotundus rhizomes. Facing co-eluting peaks in k samples, a trilinear structure was reconstructed to obtain the second-order advantage. The retention time (RT) shift with multi-channel detection signals for different samples has been vital in maintaining the trilinear structure, thus a modified multiscale peak alignment (mMSPA) method was proposed in this paper. The peak position and peak width of representative ion profile were firstly detected by mMSPA using Continuous Wavelet Transform with Haar wavelet as the mother wavelet (Haar CWT). Then, the raw shift was confirmed by Fast Fourier Transform (FFT) cross correlation calculation. To obtain the optimal shift, Haar CWT was again used to detect the subtle deviations and be amalgamated in calculation. Here, to ensure there is no peaks shape alternation, the alignment was performed in local domains of data matrices, and all data points in the peak zone were moved via linear interpolation in non-peak parts. Finally, chemical components of interest in Ligusticum chuanxiong Hort - Cyperus rotundus rhizomes were analyzed by HS-SPME-GCMS and mMSPA-alternating trilinear decomposition (ATLD) resolution. As a result, the concentration variation between herbs and their pharmaceutical products can provide a scientific basic for the quality standard establishment of traditional Chinese medicines.


