Biochanin ACAS# 491-80-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 491-80-5 | SDF | Download SDF |
| PubChem ID | 5280373 | Appearance | Yellowish powder |
| Formula | C16H12O5 | M.Wt | 284.26 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 4-Methylgenistein; Olmelin | ||
| Solubility | DMSO : ≥ 50 mg/mL (175.90 mM) *"≥" means soluble, but saturation unknown. | ||
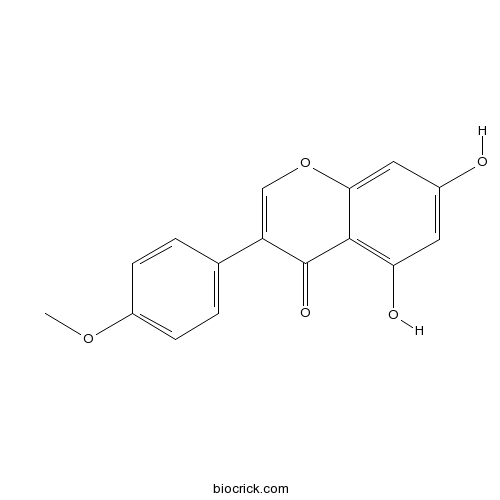
| Chemical Name | 5,7-dihydroxy-3-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=COC3=CC(=CC(=C3C2=O)O)O | ||
| Standard InChIKey | WUADCCWRTIWANL-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Biochanin A, an O-methylated natural isoflavonoid classified as phytoestrogen, is a naturally occurring fatty acid amide hydrolase (FAAH) inhibitor, which inhibits FAAH with IC50s of 1.8, 1.4 and 2.4 μM for mouse, rat, and human FAAH, respectively. Biochanin A has hypoglycemic, antilipemic,anti-tumorigenesis, anti-oxidation, and anti-inflammatory properties, it also has neuroprotective effects in cerebral ischemia/reperfusion by inhibiting inflammatory response and the inactivation of p38 signaling pathway. Biochanin A could inhibit Methicillin-resistant Staphylococcus aureus efflux system through reducing pathogen' s expression of nor A and norA protein. |
| Targets | TNF-α | IL Receptor | p38MAPK | LDL | Bcl-2/Bax | P-gp | EGFR | NO | NOS | IkB | NF-kB | IKK | FAAH |
| In vitro | [Inhibitory effects of biochanin A on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA)].[Pubmed: 25803898]Wei Sheng Wu Xue Bao. 2014 Oct 4;54(10):1204-11.
Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation.[Pubmed: 21147093 ]Eur J Pharmacol. 2011 Feb 25;653(1-3):8-15.Biochanin A, an isoflavone, existing in red clover, cabbage and alfalfa, has an inhibitory and apoptogenic effect on certain cancer cells. However, the actual mechanism by which this compound inhibits proliferation and induces apoptosis in cancer cells and the mechanism of its anti-inflammatory activities have not been well characterized. In this study, we have investigated the anti-inflammatory and anti-proliferative activity of Biochanin A. |
| In vivo | Effect of biochanin a on serum visfatin level of streptozocin-induced diabetic rats.[Pubmed: 25593725]Iran Red Crescent Med J. 2014 Sep 5;16(9):e15424.Bioflavonoids are well known for their multi directional biologic activity including antidiabetic effect. It has been demonstrated that flavonoids can act as insulin secretagogue or insulin mimetic agents.
This experimental study was designed in Arak University of Medical Sciences, Arak, Iran, to investigate the effects of Biochanin A (a bioflavonoid) on fasting blood glucose (FBG), body weight, glycosylated hemoglobin (HbA1c), lipid profile, serum enzymes, and visfatin of streptozocin-induced diabetic rats.
|
| Kinase Assay | Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation.[Pubmed: 8497428]Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport.[Pubmed: 12604704 ]J Pharmacol Exp Ther. 2003 Mar;304(3):1258-67.Flavonoids are constituents of fruits, vegetables, and plant-derived beverages, as well as components in herbal-containing dietary supplements. The objective of this investigation was to characterize the effect of flavonoids on P-glycoprotein (P-gp)-mediated cellular efflux and to determine the molecular mechanism(s) of the flavonoid-drug interaction.
Prostate. 1993;22(4):335-45.
The effect of the isoflavones, genistein, daidzein, and Biochanin A on the growth of the LNCaP and DU-145 human prostate cancer cell lines has been examined. |
| Cell Research | Biochanin A promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in breast cancer cells.[Pubmed: 25613180]Cell Physiol Biochem. 2015;35(2):639-46.Biochanin A and formononetin are O-methylated isoflavones that are isolated from the root of Astragalus membranaceus, and have antitumorigenic effects. Our previous studies found that formononetin triggered growth-inhibitory and apoptotic activities in MCF-7 breast cancer cells. We performed in vivo and in vitro studies to further investigate the potential effect of Biochanin A in promoting cell proliferation in estrogen receptor (ER)-positive cells, and to elucidate underlying mechanisms. |
| Animal Research | Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses.[Pubmed: 25466482]J Neurol Sci. 2015 Jan 15;348(1-2):121-5.Biochanin A, an O-methylated natural isoflavonoid classified as phytoestrogen, has been reported to show anti-tumorigenesis, anti-oxidation, and anti-inflammatory properties. However, little is known about the effects of Biochanin A on cerebral ischemia/reperfusion.
|

Biochanin A Dilution Calculator

Biochanin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5179 mL | 17.5895 mL | 35.1791 mL | 70.3581 mL | 87.9477 mL |
| 5 mM | 0.7036 mL | 3.5179 mL | 7.0358 mL | 14.0716 mL | 17.5895 mL |
| 10 mM | 0.3518 mL | 1.759 mL | 3.5179 mL | 7.0358 mL | 8.7948 mL |
| 50 mM | 0.0704 mL | 0.3518 mL | 0.7036 mL | 1.4072 mL | 1.759 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3518 mL | 0.7036 mL | 0.8795 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Biochanin A is a naturally occurring fatty acid amide hydrolase (FAAH) inhibitor, which inhibits FAAH with IC50s of 1.8, 1.4 and 2.4 μM for mouse, rat, and human FAAH, respectively.
In Vitro:Biochanin A inhibits the hydrolysis of 0.5 µM AEA by mouse, rat and human FAAH with IC50 s of 1.8, 1.4 and 2.4 µM respectively. FAAH is inhibited by Biochanin A with a pIC50 value of 6.21±0.02, corresponding to an IC50 value of 0.62 µM. Biochanin A produces significant inhibition of the URB597-sensitive tritium retention at high nanomolar-low micromolar concentrations. Experiments are run with human FAAH and 0.5 µM [3H]AEA with assay conditions giving these higher utilization rates, the activity is still inhibited by Biochanin A, Genistein, Formononetin and Daidzein in the low micromolar range (IC50s of 6.0, 8.4, 12 and 30 µM, respectively)[1].
In Vivo:Biochanin A is tested at doses of 30, 100 and 300 µg. The highest dose also reduced formalin-induced ERK phosphorylation in a manner antagonized by AM251. Thus, Biochanin A behaved like URB597 after local administration to the paw. In anaesthetized mice, URB597 (30 µg i.pl.) and Biochanin A (100 µg i.pl.) both inhibit the spinal phosphorylation of extracellular signal-regulated kinase produced by the intraplantar injection of formalin. The effects of both compounds are significantly reduced by the CB1 receptor antagonist/inverse agonist AM251 (30 µg i.pl.). Biochanin A (15 mg/k i.v.) does not increase brain AEA concentrations, but produces a modest potentiation of the effects of 10 mg/kg i.v. AEA in the tetrad test. Biochanin A (15 mg/kg i.v.) is without effects on its own, but significantly potentiates the effects of AEA (10 mg/kg i.v.)[1].
References:
[1]. Thors L, et al. Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br J Pharmacol. 2010 Jun;160(3):549-60.
- Iridin
Catalog No.:BCN6868
CAS No.:491-74-7
- Chrysoeriol
Catalog No.:BCN5601
CAS No.:491-71-4
- Luteolin
Catalog No.:BCN5600
CAS No.:491-70-3
- Isosakuranin
Catalog No.:BCN3712
CAS No.:491-69-0
- Baicalein
Catalog No.:BCN5599
CAS No.:491-67-8
- Kaempferide
Catalog No.:BCN5598
CAS No.:491-54-3
- Quercetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN1261
CAS No.:491-50-9
- 4-HQN
Catalog No.:BCC2448
CAS No.:491-36-1
- Valeroidine
Catalog No.:BCN1920
CAS No.:490-96-0
- Gentisic acid
Catalog No.:BCN3408
CAS No.:490-79-9
- 2,5-Dihydroxyacetophenone
Catalog No.:BCN3825
CAS No.:490-78-8
- Homoquinolinic acid
Catalog No.:BCC6570
CAS No.:490-75-5
- Hemokinin 1 (human)
Catalog No.:BCC5923
CAS No.:491851-53-7
- TCS PIM-1 1
Catalog No.:BCC2447
CAS No.:491871-58-0
- Thermopsidine
Catalog No.:BCN7923
CAS No.:492-02-4
- (+)-Sparteine
Catalog No.:BCC9249
CAS No.:492-08-0
- Plathymenin
Catalog No.:BCN6810
CAS No.:492-12-6
- Butin
Catalog No.:BCN4630
CAS No.:492-14-8
- Kynurenic acid
Catalog No.:BCN2228
CAS No.:492-27-3
- α-D-Glucose
Catalog No.:BCC9197
CAS No.:492-62-6
- Savinin
Catalog No.:BCN5602
CAS No.:493-95-8
- Methyl L-pyroglutamate
Catalog No.:BCN7060
CAS No.:4931-66-2
- Nifuratel
Catalog No.:BCC1800
CAS No.:4936-47-4
- Isoammodendrine
Catalog No.:BCN2146
CAS No.:494-15-5
Biochanin A promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in breast cancer cells.[Pubmed:25613180]
Cell Physiol Biochem. 2015;35(2):639-46.
BACKGROUND: Biochanin A and formononetin are O-methylated isoflavones that are isolated from the root of Astragalus membranaceus, and have antitumorigenic effects. Our previous studies found that formononetin triggered growth-inhibitory and apoptotic activities in MCF-7 breast cancer cells. We performed in vivo and in vitro studies to further investigate the potential effect of Biochanin A in promoting cell proliferation in estrogen receptor (ER)-positive cells, and to elucidate underlying mechanisms. METHODS: ERalpha-positive breast cancer cells (T47D, MCF-7) were treated with Biochanin A. The MTT assay and flow cytometry were used to assess cell proliferation and apoptosis. mRNA levels of ERalpha, Bcl-2, and miR-375 were quantified using real-time polymerase chain reaction. Compared with the control, low Biochanin A concentrations (2-6 muM) stimulated ERalpha-positive cell proliferation (T47D, MCF-7). The more sensitive T47D cells were used to study the relevant signaling pathway. RESULTS: After treatment with Biochanin A, ERalpha, miR-375, and Bcl-2 expression was significantly upregulated. Additionally, in the in vivo studies, uterine weight in ovariectomized mice treated with Biochanin A increased significantly. CONCLUSION: This study demonstrated that Biochanin A promoted ERalpha-positive cell proliferation through miR-375 activation and this mechanism is possibly involving in a miR-375 and ERalpha feedback loop.
Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport.[Pubmed:12604704]
J Pharmacol Exp Ther. 2003 Mar;304(3):1258-67.
Flavonoids are constituents of fruits, vegetables, and plant-derived beverages, as well as components in herbal-containing dietary supplements. The objective of this investigation was to characterize the effect of flavonoids on P-glycoprotein (P-gp)-mediated cellular efflux and to determine the molecular mechanism(s) of the flavonoid-drug interaction. Studies were conducted in the sensitive and multidrug resistant human breast cancer cell lines MCF-7 and MDA435/LCC6 and examined the effects of the flavonoids Biochanin A, morin, phloretin, and silymarin on daunomycin (DNM) accumulation and doxorubicin cytotoxicity. The potential mechanism(s) involved in the interaction was evaluated by determining flavonoid effects on 1) P-gp ATPase activity, 2) [(3)H]azidopine photoaffinity labeling of P-gp, and 3) cellular P-gp levels. The flavonoids increased [(3)H]DNM accumulation in P-gp positive cells, but not P-gp negative cells, and these effects were both flavonoid concentration- and P-gp expression level-dependent. Biochanin A and silymarin potentiated doxorubicin cytotoxicity in P-gp positive cells. Biochanin A and phloretin stimulated, whereas morin and silymarin inhibited P-gp ATPase activity, confirming that these flavonoids interact with P-gp. Morin and silymarin significantly inhibited [(3)H]azidopine photoaffinity labeling of P-gp, suggesting a direct interaction with P-gp substrate binding. A 24-h preincubation with all flavonoids, followed by flavonoid removal, did not alter cellular P-gp level in P-gp positive cells. In conclusion, Biochanin A, morin, phloretin, and silymarin all inhibited P-gp-mediated cellular efflux and the mechanism of the interaction involved, at least in part, a direct interaction. The findings of this study indicate a potential for significant flavonoid-drug interactions with P-gp substrates.
Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses.[Pubmed:25466482]
J Neurol Sci. 2015 Jan 15;348(1-2):121-5.
Biochanin A, an O-methylated natural isoflavonoid classified as phytoestrogen, has been reported to show anti-tumorigenesis, anti-oxidation, and anti-inflammatory properties. However, little is known about the effects of Biochanin A on cerebral ischemia/reperfusion. In this study, the neuroprotective and anti-inflammatory effects of Biochanin A against ischemia/reperfusion injury, as well as the related molecular mechanisms, were investigated in rat models. Male Sprague-Dawley rats were subjected to middle cerebral artery occlusion (MCAO) for 2h, followed by 24h of reperfusion. Then neurological deficits, infarct volume and brain edema were evaluated. The MPO activity and TNF-alpha and IL-1beta levels in ischemic boundary zone were determined by a spectrophotometer and the enzyme-linked immunosorbent assay (ELISA). The expressions of TNF-alpha, IL-1beta, and phosphorylation of p38 were measured by RT-PCR or Western blotting. Consequently, our findings showed that Biochanin A treatment for 14 days had significantly reduced infarct volume and brain edema, and improved neurological deficits in focal cerebral ischemia/reperfusion rats. The MPO activity and TNF-alpha and IL-1beta levels were greatly increased after ischemia/reperfusion injury, while treatment with Biochanin A dramatically suppressed these inflammatory processes. Furthermore, Biochanin A attenuated the increase in p-p38 level in the ischemia/reperfusion brain tissue. Taken together, Biochanin A has been shown to have neuroprotective effects in cerebral ischemia/reperfusion, and the mechanisms may correlate with inhibiting inflammatory response, as well as the inactivation of p38 signaling pathway.
Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation.[Pubmed:8497428]
Prostate. 1993;22(4):335-45.
The effect of the isoflavones, genistein, daidzein, and Biochanin A on the growth of the LNCaP and DU-145 human prostate cancer cell lines has been examined. Genistein and Biochanin A, but not daidzein, inhibit both serum and EGF-stimulated growth of LNCaP and DU-145 cells (IC50 values from 8.0 to 27 micrograms/ml for serum and 4.3 to 15 micrograms/ml for EGF), but have no significant effect of the EGF receptor tyrosine autophosphorylation. In contrast, tyrphostin 25, a specific EGF receptor tyrosine kinase inhibitor, inhibits EGF-stimulated growth and EGF receptor tyrosine autophosphorylation in these whole cells, but does not inhibit serum-stimulated growth. These data suggest that the mechanism of action of genistein and Biochanin A does not depend on inhibition of EGF receptor tyrosine autophosphorylation, but on a more distal event in the EGF receptor-mediated signal transduction cascade.
Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFkappaB nuclear translocation.[Pubmed:21147093]
Eur J Pharmacol. 2011 Feb 25;653(1-3):8-15.
Biochanin-A, an isoflavone, existing in red clover, cabbage and alfalfa, has an inhibitory and apoptogenic effect on certain cancer cells. However, the actual mechanism by which this compound inhibits proliferation and induces apoptosis in cancer cells and the mechanism of its anti-inflammatory activities have not been well characterized. In this study, we have investigated the anti-inflammatory and anti-proliferative activity of Biochanin-A. The effects of Biochanin-A on RAW 264.7, HT-29 cell lines and mouse peritoneal macrophages have been investigated in vitro. Cell proliferation and anti-inflammatory effects were analyzed by 3-(4-5-dimethylthiozol-2-yl)2-5-diphenyl-tetrazolium bromide (MTT) assay, (3)H-thymidine incorporation assay, Western blot, cytokines estimation, Luciferase assay, Electrophoretic mobility shift assay (EMSA) and Kinase assay. Present investigation demonstrated that, Biochanin-A inhibited lipopolysacharide (LPS)-induced nitric oxide(NO) production in macrophage and showed dose dependent inhibition of inducible nitric oxide synthase (iNOS) expression. The induction of NF-kappaB binding activity by LPS was inhibited markedly by co-incubation with different doses of Biochanin-A. Biochanin-A inhibited the LPS-induced IkB kinase (IKK) activity and nuclear factor kappa beta (NF-kappaB) activation associated with the inhibition of iNOS expression. LPS-induced phosphorylation of IkappaBalpha and p38 MAPK was blocked by Biochanin-A and it inhibited IL-6, IL-1beta and TNF-alpha production in RAW264.7 cells indicating its anti-inflammatory activity in association with anti-proliferation. Biochanin-A is important for the prevention of phosphorylation and degradation of IkappaBalpha, thereby blocking NF-kappaB activation, which in turn leads to decreased expression of the iNOS, thus preventing proliferation and inflammation.
Effect of biochanin a on serum visfatin level of streptozocin-induced diabetic rats.[Pubmed:25593725]
Iran Red Crescent Med J. 2014 Sep 5;16(9):e15424.
BACKGROUND: Bioflavonoids are well known for their multi directional biologic activity including antidiabetic effect. It has been demonstrated that flavonoids can act as insulin secretagogue or insulin mimetic agents. OBJECTIVES: This experimental study was designed in Arak University of Medical Sciences, Arak, Iran, to investigate the effects of Biochanin A (a bioflavonoid) on fasting blood glucose (FBG), body weight, glycosylated hemoglobin (HbA1c), lipid profile, serum enzymes, and visfatin of streptozocin-induced diabetic rats. PATIENTS AND METHODS: We used 24 male Wistar rats and randomly allocated them to four groups of six rats. One group was randomly assigned as control and diabetes was induced in three other groups by administration of streptozocin (35 mg/kg of body weight) intraperitoneally. The groups received the following treatments: group 1 (control), 5% DMSO; group 2 (diabetic control), 0.5% DMSO; and group 3 and 4, respectively 10 and 15 mg/kg Biochanin A for 30 days. Body weight and biochemical parameters including FBG, HbA1c, lipid profile, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and visfatin were measured in all rats. RESULTS: FBG level was significantly reduced in treated diabetic rats (139.8 +/- 9.3 and 206 +/- 11 mg/dL in groups 3 and 4, respectively) in comparison to the diabetic control (295.1 +/- 14 mg/dL) (P < 0.05). Administration of Biochanin A significantly decreased HbA1c in group 3 (6.66 +/- 0.33) and group 4 (7.11 +/- 0.31) in comparison to the diabetic control group (8.26 +/- 0.44) (P < 0.05). Levels of serum visfatin were improved to near normal levels in the treated rats (249 +/- 35.5 and 161.33 +/- 13.07 in groups 3 and 4, respectively) in comparison to the diabetic control (302.17 +/- 19.4) (P < 0.05). Furthermore, Biochanin A showed a protective effect against weight loss in diabetic rats (P < 0.05). In treated rats, serum total cholesterol, triglyceride, and low-density lipoprotein cholesterol (LDL-c) were significantly decreased and high-density lipoprotein (HDL-c) was increased in comparison with the diabetic control group. In addition, Biochanin A restored the altered plasma enzymes (AST, ALT, and ALP) activities to near normal. Histopathologic examination of the pancreas also indicated that Biochanin A had protective effects on beta-cells in streptozocin-induced diabetic rats. CONCLUSIONS: This study demonstrated that Biochanin A possessed hypoglycemic and antilipemic activities and could increase visfatin expression, which suggests its beneficial effect in the treatment of diabetes.
[Inhibitory effects of biochanin A on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA)].[Pubmed:25803898]
Wei Sheng Wu Xue Bao. 2014 Oct 4;54(10):1204-11.
OBJECTIVE: To study the inhibitory effect of Biochanin A on efflux system of Methicillin-resistant Staphylococcus aureus (MRSA). METHODS: Inhibitory effects of Biochanin A on efflux system of Strain MRSA41577 were evaluated using double dilution method, two plate method and fluorescence spectrophotometry. Real time PCR and SDS-PAGE were applied to detect the expression of MRSA41577 norA and to analyze the changes of MRSA41577 efflux protein before and after dosing Biochanin A in association with liquid chromatography mass spectrometry to determinate protein variation. RESULTS: Biochanin A alone had no inhibitory effect on MRSA41577, but it showed synergy effect with ciprofloxacin in inhibition MRSA41577 in which 40pg/mL Biochanin A decreased the minimum inhibitory concentration (MIC) value of ciprofloxacin from 64 microg/mL to 8 microgg/mL. Biochanin A significantly increased the accumulation of ciprofloxacin in MRSA41577 in a time-dependent manner. At 15 min, Biochanin A increased ciprofloxacin in MRSA41577 by 83%, which is similar to that of reserpine (positive control). Further mechanism studies indicated that Biochanin A could reduce the expression of nor A in ciprofloxacin-treated MRSA41577. After incubated with Biochanin A and ciprofloxacin for 16 h, the relative expression of nor A of MRSA41577 was reduced by 65%. SDS-PAGE analysis showed that the total protein profiles of MRSA41577 were significantly changed after treatment with Biochanin A for 16h, in which both norA protein and efflux system ABC transporter ATP-binding protein were significantly decreased. CONCLUSION: Biochanin A could inhibit Methicillin-resistant Staphylococcus aureus efflux system through reducing pathogen' s expression of nor A and norA protein.


