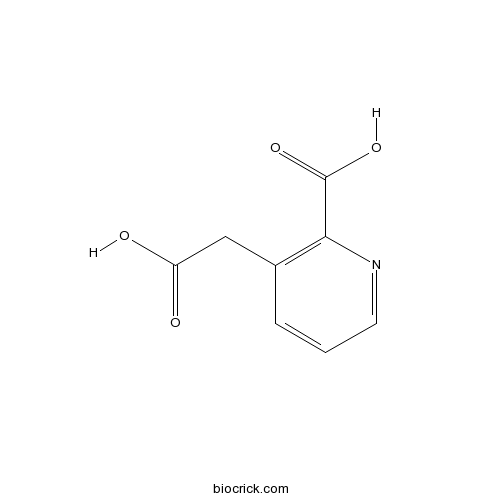
Homoquinolinic acidCAS# 490-75-5 |
- Hoechst 33342 analog 2
Catalog No.:BCC1631
CAS No.:106050-84-4
- Hoechst 33342
Catalog No.:BCC1629
CAS No.:23491-52-3
- Hoechst 33258 analog 2
Catalog No.:BCC1625
CAS No.:23491-54-5
- Hoechst 33258 analog 5
Catalog No.:BCC1627
CAS No.:23491-55-6
- Hoechst 34580
Catalog No.:BCC1632
CAS No.:23555-00-2
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 490-75-5 | SDF | Download SDF |
| PubChem ID | 3080554 | Appearance | Powder |
| Formula | C8H7NO4 | M.Wt | 181.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
| Chemical Name | 3-(carboxymethyl)pyridine-2-carboxylic acid | ||
| SMILES | C1=CC(=C(N=C1)C(=O)O)CC(=O)O | ||
| Standard InChIKey | HQPMJFFEXJELOQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H7NO4/c10-6(11)4-5-2-1-3-9-7(5)8(12)13/h1-3H,4H2,(H,10,11)(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent NMDA receptor agonist; subtype-selective. |

Homoquinolinic acid Dilution Calculator

Homoquinolinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.5203 mL | 27.6014 mL | 55.2029 mL | 110.4057 mL | 138.0072 mL |
| 5 mM | 1.1041 mL | 5.5203 mL | 11.0406 mL | 22.0811 mL | 27.6014 mL |
| 10 mM | 0.552 mL | 2.7601 mL | 5.5203 mL | 11.0406 mL | 13.8007 mL |
| 50 mM | 0.1104 mL | 0.552 mL | 1.1041 mL | 2.2081 mL | 2.7601 mL |
| 100 mM | 0.0552 mL | 0.276 mL | 0.552 mL | 1.1041 mL | 1.3801 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gaultherin
Catalog No.:BCN2482
CAS No.:490-67-5
- Epicatechin
Catalog No.:BCN5597
CAS No.:490-46-0
- Robinetin
Catalog No.:BCN5596
CAS No.:490-31-3
- Beta-Tocotrienol
Catalog No.:BCN3725
CAS No.:490-23-3
- a-Truxilline
Catalog No.:BCN1947
CAS No.:490-17-5
- SBC-115076
Catalog No.:BCC6440
CAS No.:489415-96-5
- ML 213
Catalog No.:BCC6213
CAS No.:489402-47-3
- Guaiol
Catalog No.:BCN6619
CAS No.:489-86-1
- Guaiazulen
Catalog No.:BCC8180
CAS No.:489-84-9
- Globulol
Catalog No.:BCN6901
CAS No.:489-41-8
- Gossypetin
Catalog No.:BCN8075
CAS No.:489-35-0
- Limocitrin
Catalog No.:BCN3346
CAS No.:489-33-8
- 2,5-Dihydroxyacetophenone
Catalog No.:BCN3825
CAS No.:490-78-8
- Gentisic acid
Catalog No.:BCN3408
CAS No.:490-79-9
- Valeroidine
Catalog No.:BCN1920
CAS No.:490-96-0
- 4-HQN
Catalog No.:BCC2448
CAS No.:491-36-1
- Quercetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN1261
CAS No.:491-50-9
- Kaempferide
Catalog No.:BCN5598
CAS No.:491-54-3
- Baicalein
Catalog No.:BCN5599
CAS No.:491-67-8
- Isosakuranin
Catalog No.:BCN3712
CAS No.:491-69-0
- Luteolin
Catalog No.:BCN5600
CAS No.:491-70-3
- Chrysoeriol
Catalog No.:BCN5601
CAS No.:491-71-4
- Iridin
Catalog No.:BCN6868
CAS No.:491-74-7
- Biochanin A
Catalog No.:BCN1224
CAS No.:491-80-5
Ethanol differentially inhibits homoquinolinic acid- and NMDA-induced neurotoxicity in primary cultures of cerebellar granule cells.[Pubmed:12834259]
Neurochem Res. 2003 Aug;28(8):1193-9.
The potency of ethanol to inhibit N-methyl-D-aspartate (NMDA) receptor functions may depend on the subunit composition of the NMDA receptors. We used a NR2A-B subunit-selective NMDA receptor agonist, Homoquinolinic acid (HQ), and a subunit-unselective agonist, NMDA, to induce neurotoxicity in cerebellar granule cells and examined the neuroprotective actions of ethanol, as well as NR2A- and NR2B-subunit selective antagonists, respectively. HQ was a more potent neurotoxic agent than NMDA, as measured by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. NR2A- and NR2B-selective NMDA receptor antagonists displayed quite similar neuroprotective potencies against the NMDA- and HQ-produced cell death, indicating that the higher potency of HQ to induce neurotoxicity cannot be simply explained by NR2A- or NR2B-subunit selectivity. As expected, ethanol (25 and 50 mM) attenuated the NMDA-induced neurotoxicity in a non-competitive manner by significantly reducing the maximum neurotoxicity produced by NMDA. By contrast, ethanol inhibited the HQ-induced neurotoxicity in a manner resembling a competitive-like interaction significantly increasing the EC50 value for HQ, without reducing the maximum neurotoxicity produced by HQ. These results suggest that HQ reveals either a novel site or a not previously observed mechanism of interaction between ethanol and NMDA receptors in rat cerebellar granule cell cultures.
The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits.[Pubmed:8740453]
Neurochem Int. 1996 Apr;28(4):445-52.
Quinolinic acid is an endogenous neurotoxin with NMDA receptor agonist properties. As such it may be the etiologic agent in many diseases. In this paper the NMDA receptor agonist properties of quinolinic acid, as well as those of Homoquinolinic acid, a non endogenous analogue, were investigated in Xenopus oocytes injected with 12-day-old rat cortical mRNA or with recombinant NMDA receptors. In oocytes injected with cortical mRNA, quinolinic acid was a weak NMDA receptor agonist: millimolar concentrations were necessary to induce responses that were smaller than maximal responses induced by NMDA; Homoquinolinic acid and NMDA had similar affinities but different efficacies: maximal responses induced by Homoquinolinic acid were larger than maximal responses induced by NMDA. Cortical mRNA, as verified by RT-PCR and restriction analysis, contains various NMDA subunits. In order to investigate if the low affinity or efficacy of quinolinic acid could be explained by receptor composition, the pharmacological properties of the putative agonists were investigated in oocytes expressing binary combinations of recombinant NMDA receptors. Quinolinic acid did not activate receptors containing NR1 + NR2C but did activate receptors containing NR1 + NR2A and NR1 + NR2B even if only at millimolar concentrations; Homoquinolinic acid activated all subunit combinations but was less efficient than NMDA only in the NR1 + NR2C subunit combination. The relative efficacies of quinolinic acid and Homoquinolinic acid were evaluated by comparing the maximal responses induced by these agonists with those induced by NMDA and glutamate in the same oocytes. The rank order of potency was quinolinic acid < NMDA < Homoquinolinic acid < or = glutamate for the NR1 + NR2A and NR1 + NR2B combinations whereas for NR1 + NR2C it was quinolinic acid << << Homoquinolinic acid < NMDA < or = glutamate. The use of quinolinic acid and Homoquinolinic acid may thus help to identify endogenous receptors containing the NR2C subunit.
Structure/activity relations of N-methyl-D-aspartate receptor ligands as studied by their inhibition of [3H]D-2-amino-5-phosphonopentanoic acid binding in rat brain membranes.[Pubmed:2901691]
Neuroscience. 1988 Jul;26(1):17-31.
Structure/activity relations of agonists and antagonists for the N-methyl-D-aspartate receptor have been investigated by measuring the ability of a large range of substances to inhibit binding of [3H]2-amino-5-phosphonopentanoate to rat brain membranes. A major difference between optimum structures for agonist and antagonist activity lay in the differential effectiveness of sulphonic and phosphonic acid groups as the omega-acidic terminal in these two types of compound. The sulphonic acid moiety was an effective omega-acidic terminal in short chain agonists, but not in longer chain antagonists, while the phosphonic acid group was the most effective omega-acidic terminal in longer chain antagonists, but was only very weakly active in short chain agonists. It is proposed that the binding site of the omega-acidic terminal of antagonists is different from that for the omega-acidic group of agonists. Other structural features conducive to effective interaction of ligands with the receptor are discussed.
Excitant activity of methyl derivatives of quinolinic acid on rat cortical neurones.[Pubmed:6546701]
Br J Pharmacol. 1984 Jan;81(1):175-81.
Various synthetic analogues of quinolinic acid have been tested for agonist and antagonist properties when applied by microiontophoresis to neurones in the rat cerebral cortex. Quinolinic acid 2-methylester was a weak antagonist of N-methyl-D-aspartate (NMDA) and quinolinic acid, but also showed agonist activity, being about half as active as quinolinic acid. The excitant effects of the compound could be antagonized by the NMDA receptor blocker, 2-amino-7-phosphonoheptanoic acid (2APH). N-methyl-quinolinic acid 2, 3-dimethylester showed very weak agonist and antagonist activity. Homoquinolinic acid was a potent excitant of cortical neurones, being about five times more active than quinolinic acid and approximately equipotent with NMDA. The excitations were blocked by 2APH or its pentanoate analogue (2APV). Homoquinolinic acid 2-methylester was also active as an agonist. N-methyl-DL-glutamic acid was also tested, since Homoquinolinic acid is a rigid analogue of this compound. Although it did cause excitation of 5 of the 16 units tested, N-methyl-glutamate was a weaker agonist than NMDA or homoquinolinate. Phthalic acid, ejected as an anion caused excitation of 14 out of 16 units. It is therefore concluded that the ring nitrogen of quinolinic acid is not essential for excitant activity. Since Homoquinolinic acid is a rigid analogue of glutamic acid, but causes excitation by acting apparently on the NMDA receptor, the results are consistent with the suggestion that activation of the NMDA receptor may require the carboxyl groups to be held in a relatively extended configuration.


