ThermopsidineCAS# 492-02-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 492-02-4 | SDF | Download SDF |
| PubChem ID | 91885396 | Appearance | Powder |
| Formula | C24H30N4O2 | M.Wt | 406.52 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
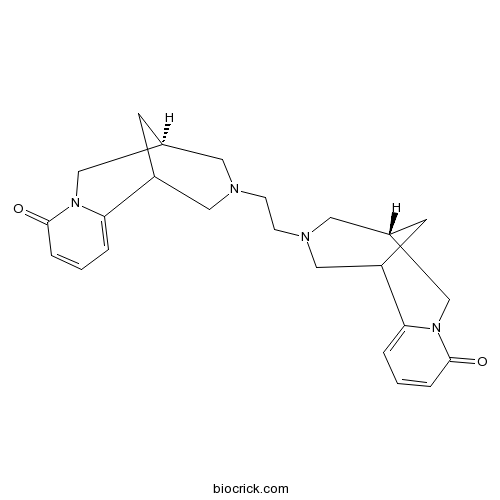
| SMILES | C1C2CN(CC1C3=CC=CC(=O)N3C2)CCN4CC5CC(C4)C6=CC=CC(=O)N6C5 | ||
| Standard InChIKey | LEXDAVFCJPDCNA-VCAKUFKGSA-N | ||
| Standard InChI | InChI=1S/C24H30N4O2/c29-23-5-1-3-21-19-9-17(13-27(21)23)11-25(15-19)7-8-26-12-18-10-20(16-26)22-4-2-6-24(30)28(22)14-18/h1-6,17-20H,7-16H2/t17-,18-,19?,20?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Thermopsidine Dilution Calculator

Thermopsidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4599 mL | 12.2995 mL | 24.599 mL | 49.1981 mL | 61.4976 mL |
| 5 mM | 0.492 mL | 2.4599 mL | 4.9198 mL | 9.8396 mL | 12.2995 mL |
| 10 mM | 0.246 mL | 1.23 mL | 2.4599 mL | 4.9198 mL | 6.1498 mL |
| 50 mM | 0.0492 mL | 0.246 mL | 0.492 mL | 0.984 mL | 1.23 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.246 mL | 0.492 mL | 0.615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TCS PIM-1 1
Catalog No.:BCC2447
CAS No.:491871-58-0
- Hemokinin 1 (human)
Catalog No.:BCC5923
CAS No.:491851-53-7
- Biochanin A
Catalog No.:BCN1224
CAS No.:491-80-5
- Iridin
Catalog No.:BCN6868
CAS No.:491-74-7
- Chrysoeriol
Catalog No.:BCN5601
CAS No.:491-71-4
- Luteolin
Catalog No.:BCN5600
CAS No.:491-70-3
- Isosakuranin
Catalog No.:BCN3712
CAS No.:491-69-0
- Baicalein
Catalog No.:BCN5599
CAS No.:491-67-8
- Kaempferide
Catalog No.:BCN5598
CAS No.:491-54-3
- Quercetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN1261
CAS No.:491-50-9
- 4-HQN
Catalog No.:BCC2448
CAS No.:491-36-1
- Valeroidine
Catalog No.:BCN1920
CAS No.:490-96-0
- (+)-Sparteine
Catalog No.:BCC9249
CAS No.:492-08-0
- Plathymenin
Catalog No.:BCN6810
CAS No.:492-12-6
- Butin
Catalog No.:BCN4630
CAS No.:492-14-8
- Kynurenic acid
Catalog No.:BCN2228
CAS No.:492-27-3
- α-D-Glucose
Catalog No.:BCC9197
CAS No.:492-62-6
- Savinin
Catalog No.:BCN5602
CAS No.:493-95-8
- Methyl L-pyroglutamate
Catalog No.:BCN7060
CAS No.:4931-66-2
- Nifuratel
Catalog No.:BCC1800
CAS No.:4936-47-4
- Isoammodendrine
Catalog No.:BCN2146
CAS No.:494-15-5
- Nornicotine
Catalog No.:BCN8176
CAS No.:494-97-3
- TC-G 1001
Catalog No.:BCC6316
CAS No.:494191-73-0
- Ginsenoside Rk1
Catalog No.:BCN3552
CAS No.:494753-69-4
Long-term exposure to the new nicotinic antagonist 1,2-bisN-cytisinylethane upregulates nicotinic receptor subtypes of SH-SY5Y human neuroblastoma cells.[Pubmed:16273122]
Br J Pharmacol. 2005 Dec;146(8):1096-109.
Nicotinic drug treatment can affect the expression of neuronal nicotinic acetylcholine receptors (nAChR) both in vivo and in vitro through molecular mechanisms not fully understood. The present study investigated the effect of the novel cytisine dimer 1,2-bisN-cytisinylethane (CC4) on nAChR natively expressed by SH-SY5Y neuroblastoma cells in culture. CC4 lacked the agonist properties of cytisine and was a potent antagonist (IC50=220 nM) on nAChRs. Chronic treatment of SH-SY5Y cells with 1 mM CC4 for 48 h increased the expression of 3H-epibatidine (3H-Epi; 3-4-fold) or 125I-alpha-bungarotoxin (125I-alphaBgtx; 1.2-fold) sensitive receptors present on the cell membrane and in the intracellular pool. Comparable data were obtained with nicotine or cytisine, but not with carbamylcholine, d-tubocurarine, di-hydro-beta-erythroidine or hexametonium. Immunoprecipitation and immunopurification studies showed that the increase in 3H-Epi-binding receptors was due to the enhanced expression of alpha3beta2 and alpha3beta2beta4 subtypes without changes in subunit mRNA transcription or receptor half-life. The upregulation was not dependent on agonist/antagonist properties of the drugs, and did not concern muscarinic or serotonin receptors. Whole-cell patch clamp analysis of CC4-treated cells demonstrated larger nicotine-evoked inward currents with augmented sensitivity to the blockers alpha-conotoxin MII or methyllycaconitine. In conclusion, chronic treatment with CC4 increased the number of nAChRs containing beta2 and alpha7 subunits on the plasma membrane, where they were functionally active. In the case of beta2-containing receptors, we propose that CC4, by binding to intracellular receptors, triggered a conformational reorganisation of intracellular subunits that stimulated preferential assembly and membrane-directed trafficking of beta2-containing receptor subtypes..
Nitrogen substitution modifies the activity of cytisine on neuronal nicotinic receptor subtypes.[Pubmed:12818695]
Eur J Pharmacol. 2003 Jun 20;471(2):85-96.
Cytisine very potently binds and activates the alpha 3 beta 4 and alpha 7 nicotinic subtypes, but only partially agonises the alpha 4 beta 2 subtype. Although with a lower affinity than cytisine, new cytisine derivatives with different substituents on the basic nitrogen (CC1-CC8) bind to both the heteromeric and homomeric subtypes, with higher affinity for brain [3H]epibatidine receptors. The cytisine derivatives were tested on the Ca(2+) flux of native or transfected cell lines expressing the rat alpha 7, or human alpha 3 beta 4 or alpha 4 beta 2 subtypes using Ca(2+) dynamics in conjunction with a fluorescent image plate reader. None elicited any response at doses of up to 30-100 microM, but all inhibited agonist-induced responses. Compounds CC5 and CC7 were also electrophysiologically tested on oocyte-expressed rat alpha 4 beta 2, alpha 3 beta 4 and alpha 7 subtypes. CC5 competitively antagonised the alpha 4 beta 2 and alpha 3 beta 4 subtypes with similar potency, whereas CC7 only partially agonised them with maximum responses of respectively 3% and 11% of those of 1 mM acetylcholine. Neither compound induced any current in the oocyte-expressed alpha 7 subtype, and both weakly inhibited acetylcholine-induced currents. Adding chemical groups of a different class or size to the basic nitrogen of cytisine leads to compounds that lose full agonist activity on the alpha 3 beta 4 and alpha 7 subtypes.
CC4, a dimer of cytisine, is a selective partial agonist at alpha4beta2/alpha6beta2 nAChR with improved selectivity for tobacco smoking cessation.[Pubmed:22957729]
Br J Pharmacol. 2013 Feb;168(4):835-49.
BACKGROUND AND PURPOSE: Many of the addictive and rewarding effects of nicotine are due to its actions on the neuronal nicotinic ACh receptor (nAChR) subtypes expressed in dopaminergic mesocorticolimbic cells. The partial agonists, cytisine and varenicline, are helpful smoking cessation aids. These drugs have a number of side effects that limit their usefulness. The aim of this study was to investigate the preclinical pharmacology of the cytisine dimer1,2-bisN-cytisinylethane (CC4). EXPERIMENTAL APPROACH: The effects of CC4 on nAChRs were investigated using in vitro assays and animal behaviours. KEY RESULTS: When electrophysiologically tested using heterologously expressed human subtypes, CC4 was less efficacious than cytisine on neuronal alpha4beta2, alpha3beta4, alpha7 and muscle-type receptors, and had no effect on 5-hydroxytryptamine3 receptors. Acting through alpha4beta2 and alpha6beta2 nAChRs, CC4 is a partial agonist of nAChR-mediated striatal dopamine release and, when co-incubated with nicotine, prevented nicotine's maximal effect on this response. In addition, it had low affinity for, and was less efficacious than nicotine and cytisine on the alpha3beta4 and alpha7-nAChR subtypes. Like cytisine and nicotine, CC4-induced conditioned place preference (CPP), and its self-administration shows an inverted-U dose-response curve. Pretreatment with non-reinforcing doses of CC4 significantly reduced nicotine-induced self-administration and CPP without affecting motor functions. CONCLUSION AND IMPLICATIONS: Our in vitro and in vivo findings reveal that CC4 selectively reduces behaviours associated with nicotine addiction consistent with the partial agonist selectivity of CC4 for beta2-nAChRs. The results support the possible development of CC4 or its derivatives as a promising drug for tobacco smoking cessation.


