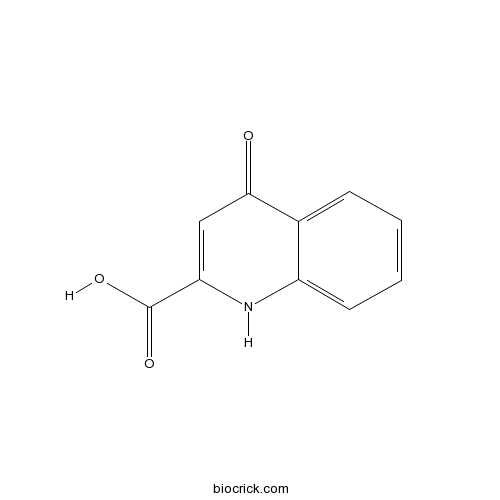
Kynurenic acidBroad spectrum EAA antagonist CAS# 492-27-3 |
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- 3-Deazaneplanocin A (DZNep) hydrochloride
Catalog No.:BCC3604
CAS No.:120964-45-6
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- PluriSIn #1 (NSC 14613)
Catalog No.:BCC2305
CAS No.:91396-88-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 492-27-3 | SDF | Download SDF |
| PubChem ID | 3845 | Appearance | White cryst. |
| Formula | C10H7NO3 | M.Wt | 189.17 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 9 mg/mL (47.58 mM; Need ultrasonic and warming) | ||
| Chemical Name | 4-oxo-1H-quinoline-2-carboxylic acid | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C=C(N2)C(=O)O | ||
| Standard InChIKey | HCZHHEIFKROPDY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H7NO3/c12-9-5-8(10(13)14)11-7-4-2-1-3-6(7)9/h1-5H,(H,11,12)(H,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Kynurenic acid, a natural metabolite of tryptophan via the kynurenine pathway, is a broad-spectrum excitatory amino acid antagonist; It proved to be an antagonist at NMDA, kainate and AMPA receptors.Kynurenic acid is the endogenous α7 nicotinic acetylcholine receptor antagonist, it has an immunomodulating effect, it can modulate amyloid-β-induced inflammation in BV-2 microglial cells. |
| Targets | Beta Amyloid | NO | TNF-α | IL Receptor | NMDAR | GPR35 |
| In vitro | The endogenous α7 nicotinic acetylcholine receptor antagonist kynurenic acid modulates amyloid-β-induced inflammation in BV-2 microglial cells.[Pubmed: 25064444]J Neurol Sci. 2014 Sep 15;344(1-2):94-9.Amyloid-β has been shown to interact with the α7 nicotinic acetylcholine receptor on neuronal cells. Not much is known on the effect on microglial cells and whether this effect can be modulated by the endogenous α7 nicotinic acetylcholine receptor antagonist Kynurenic acid. Our aim was to investigate the effect of Kynurenic acid on amyloid-β-treated BV-2 microglial cells with respect to α7 nicotinic acetylcholine receptor expression, cell viability, cytokine production and phagocytotic abilities. |
| In vivo | Pre-treatment with new kynurenic acid amide dose-dependently prevents the nitroglycerine-induced neuronal activation and sensitization in cervical part of trigemino-cervical complex.[Pubmed: 24385076]J Neural Transm. 2014 Jul;121(7):725-38.The systemic administration of nitroglycerine induces attacks in migraineurs and is able to activate and sensitize the trigeminal system in animals involving glutamate and α7-nicotinic acetylcholine receptors, among others. Kynurenic acid is one of the endogenous glutamate receptor antagonists, and exerts inhibitory action on the α7-nicotinic acetylcholine receptors. Since Kynurenic acid penetrates the blood-brain barrier poorly, therefore a newly synthesized Kynurenic acid amide, N-(2-N-pyrrolidinylethyl)-4-oxo-1H-quinoline-2-carboxamide hydrochloride (KYNAa) was used with such a side-chain substitution to facilitate brain penetration in our study. The effect of transient increases in kynurenic acid and quinolinic acid levels early in life on behavior in adulthood: Implications for schizophrenia.[Pubmed: 24091034 ]Schizophr Res. 2013 Nov;150(2-3):392-7.Kynurenic acid is a tryptophan metabolite that is synthesized and released in the brain by astrocytes and acts as an antagonist of nicotinic acetylcholine receptors and N-methyl-d-aspartate glutamate receptors, both of which are critically involved in cognition as well as neural plasticity and brain development. The concentration of Kynurenic acid is increased in the brains of persons with schizophrenia and this increase has been implicated in the cognitive and social impairments associated with the disease. In addition, growing evidence suggests that the increase in Kynurenic acid may begin early in life. For example, exposure to influenza A virus during development results in a transient increase in Kynurenic acid concentration that could disrupt normal brain development and lead to cognitive deficits later in life. Changes in Kynurenic acid may thus provide a link between developmental exposure to viruses and the increased risk of subsequently developing schizophrenia. |
| Kinase Assay | Kynurenic acid is a nutritional cue that enables behavioral plasticity.[Pubmed: 25594177]Cell. 2015 Jan 15;160(1-2):119-31.The kynurenine pathway of tryptophan metabolism is involved in the pathogenesis of several brain diseases, but its physiological functions remain unclear. |
| Cell Research | The in vitro effect of kynurenic acid on the rainbow trout (Oncorhynchus mykiss) leukocyte and splenocyte activity.[Pubmed: 25286653]Pol J Vet Sci. 2014;17(3):453-8.Kynurenic acid (KYNA), an endogenous neuroprotectant formed along the kynurenine pathway of tryptophan degradation, is a selective ligand of the GPR35 receptor, which can be found on the surface of various populations of human immune cells. In infections and inflammations, KYNA produces an anti-inflammatory effect through this receptor, by depressing the synthesis of reactive oxygen species and pro-inflammatory cytokines. However, it is still unrecognized whether receptors for Kynurenic acid are also localized on immune cells of poikilothermic animals, or whether KYNA is able to affect these cells. |

Kynurenic acid Dilution Calculator

Kynurenic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2863 mL | 26.4313 mL | 52.8625 mL | 105.725 mL | 132.1563 mL |
| 5 mM | 1.0573 mL | 5.2863 mL | 10.5725 mL | 21.145 mL | 26.4313 mL |
| 10 mM | 0.5286 mL | 2.6431 mL | 5.2863 mL | 10.5725 mL | 13.2156 mL |
| 50 mM | 0.1057 mL | 0.5286 mL | 1.0573 mL | 2.1145 mL | 2.6431 mL |
| 100 mM | 0.0529 mL | 0.2643 mL | 0.5286 mL | 1.0573 mL | 1.3216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Butin
Catalog No.:BCN4630
CAS No.:492-14-8
- Plathymenin
Catalog No.:BCN6810
CAS No.:492-12-6
- (+)-Sparteine
Catalog No.:BCC9249
CAS No.:492-08-0
- Thermopsidine
Catalog No.:BCN7923
CAS No.:492-02-4
- TCS PIM-1 1
Catalog No.:BCC2447
CAS No.:491871-58-0
- Hemokinin 1 (human)
Catalog No.:BCC5923
CAS No.:491851-53-7
- Biochanin A
Catalog No.:BCN1224
CAS No.:491-80-5
- Iridin
Catalog No.:BCN6868
CAS No.:491-74-7
- Chrysoeriol
Catalog No.:BCN5601
CAS No.:491-71-4
- Luteolin
Catalog No.:BCN5600
CAS No.:491-70-3
- Isosakuranin
Catalog No.:BCN3712
CAS No.:491-69-0
- Baicalein
Catalog No.:BCN5599
CAS No.:491-67-8
- α-D-Glucose
Catalog No.:BCC9197
CAS No.:492-62-6
- Savinin
Catalog No.:BCN5602
CAS No.:493-95-8
- Methyl L-pyroglutamate
Catalog No.:BCN7060
CAS No.:4931-66-2
- Nifuratel
Catalog No.:BCC1800
CAS No.:4936-47-4
- Isoammodendrine
Catalog No.:BCN2146
CAS No.:494-15-5
- Nornicotine
Catalog No.:BCN8176
CAS No.:494-97-3
- TC-G 1001
Catalog No.:BCC6316
CAS No.:494191-73-0
- Ginsenoside Rk1
Catalog No.:BCN3552
CAS No.:494753-69-4
- Auraptene
Catalog No.:BCN5603
CAS No.:495-02-3
- Ammijin
Catalog No.:BCN3617
CAS No.:495-30-7
- Nodakenin
Catalog No.:BCN2378
CAS No.:495-31-8
- Nodakenetin
Catalog No.:BCN5604
CAS No.:495-32-9
The in vitro effect of kynurenic acid on the rainbow trout (Oncorhynchus mykiss) leukocyte and splenocyte activity.[Pubmed:25286653]
Pol J Vet Sci. 2014;17(3):453-8.
Kynurenic acid (KYNA), an endogenous neuroprotectant formed along the kynurenine pathway of tryptophan degradation, is a selective ligand of the GPR35 receptor, which can be found on the surface of various populations of human immune cells. In infections and inflammations, KYNA produces an anti-inflammatory effect through this receptor, by depressing the synthesis of reactive oxygen species and pro-inflammatory cytokines. However, it is still unrecognized whether receptors for Kynurenic acid are also localized on immune cells of poikilothermic animals, or whether KYNA is able to affect these cells. The objective of this study has been to determine the effect of different concentrations of Kynurenic acid (12.5 microM to 10 mM) on the viability and mitogenic response of lymphocytes and on the activity of phagocytic cells isolated from blood and the spleen of rainbow trout. The results imply low toxicity of Kynurenic acid towards fish immune cells, and the proliferative effect observed at the two lowest concentrations of KYNA (12.5 microM and 25 microM) seems indicative of endogenous Kynurenic acid being capable of activating fish lymphocytes. Non-toxic, micromole concentrations of KYNA, however, had no influence on the mitogenic response of lymphocytes nor on the activity of phagocytes in rainbow trout under in vitro conditions. There is some likelihood that such an effect could be observed at lower, nanomole concentrations of KYNA.
The endogenous alpha7 nicotinic acetylcholine receptor antagonist kynurenic acid modulates amyloid-beta-induced inflammation in BV-2 microglial cells.[Pubmed:25064444]
J Neurol Sci. 2014 Sep 15;344(1-2):94-9.
Amyloid-beta has been shown to interact with the alpha7 nicotinic acetylcholine receptor on neuronal cells. Not much is known on the effect on microglial cells and whether this effect can be modulated by the endogenous alpha7 nicotinic acetylcholine receptor antagonist Kynurenic acid. Our aim was to investigate the effect of Kynurenic acid on amyloid-beta-treated BV-2 microglial cells with respect to alpha7 nicotinic acetylcholine receptor expression, cell viability, cytokine production and phagocytotic abilities. Therefore BV-2 cells were treated with oligomeric or fibrillar forms of amyloid-beta(1-40) and co-treated with Kynurenic acid. alpha7 nicotinic acetylcholine receptor quantity was investigated using Western blotting. Cell viability was assessed by staining cells with fluorescein diacetate and propidium iodide. Pro-inflammatory cytokines were measured in cell culture supernatants of treated cells with ELISAs; NO with Griess reagents and amyloid-beta uptake were investigated with fluorescence-activated cell sorting and verified by Western blotting. Amyloid-beta nor Kynurenic acid did have an effect on the protein level of the alpha7 nicotinic acetylcholine receptor. Amyloid-Beta induced cell mortality was unchanged after addition of Kynurenic acid. However, Kynurenic acid co-treatment reduced the pro-inflammatory cytokines tumour necrosis factor-alpha and IL-6 and amyloid-beta phagocytosis. We provide evidence for an immunomodulating effect of the endogenous alpha7 nicotinic acetylcholine receptor antagonist Kynurenic acid. Our findings indicate a role for Kynurenic acid in amyloid-beta associated neuroinflammation in Alzheimer disease.
Pre-treatment with new kynurenic acid amide dose-dependently prevents the nitroglycerine-induced neuronal activation and sensitization in cervical part of trigemino-cervical complex.[Pubmed:24385076]
J Neural Transm (Vienna). 2014 Jul;121(7):725-38.
The systemic administration of nitroglycerine induces attacks in migraineurs and is able to activate and sensitize the trigeminal system in animals involving glutamate and alpha7-nicotinic acetylcholine receptors, among others. Kynurenic acid is one of the endogenous glutamate receptor antagonists, and exerts inhibitory action on the alpha7-nicotinic acetylcholine receptors. Since Kynurenic acid penetrates the blood-brain barrier poorly, therefore a newly synthesized Kynurenic acid amide, N-(2-N-pyrrolidinylethyl)-4-oxo-1H-quinoline-2-carboxamide hydrochloride (KYNAa) was used with such a side-chain substitution to facilitate brain penetration in our study. We evaluated its modulatory effect on Kynurenic acid concentration in the cervical part of trigemino-cervical complex (C1-C2) and in the model of nitroglycerine-induced trigeminal activation using male Sprague-Dawley rats. One hour after 1 mmol/kg bodyweight KYNAa administration, the Kynurenic acid level increased significantly in C1-C2, which returned to the basal level at 300 min measured by high-performance liquid chromatography. KYNAa pre-treatment had dose-dependent, mitigating action on nitroglycerine-induced decrease in calcitonin gene-related peptide and increase in c-Fos, neuronal nitric oxide synthase and calmodulin-dependent protein kinase II alpha expression in the C1-C2. KYNAa also mitigated the behavioural changes after nitroglycerine. Thus, in this model KYNAa is able to modulate in a dose-dependent manner the changes in neurochemical markers of activation and sensitization of the trigeminal system directly and indirectly--via forming Kynurenic acid, possibly acting on peripheral and central glutamate or alpha7-nicotinic acetylcholine receptors. These results suggest that application of Kynurenic acid derivatives could be a useful therapeutic strategy in migraine headache in the future with a different mechanism of action.
The effect of transient increases in kynurenic acid and quinolinic acid levels early in life on behavior in adulthood: Implications for schizophrenia.[Pubmed:24091034]
Schizophr Res. 2013 Nov;150(2-3):392-7.
Kynurenic acid is a tryptophan metabolite that is synthesized and released in the brain by astrocytes and acts as an antagonist of nicotinic acetylcholine receptors and N-methyl-d-aspartate glutamate receptors, both of which are critically involved in cognition as well as neural plasticity and brain development. The concentration of Kynurenic acid is increased in the brains of persons with schizophrenia and this increase has been implicated in the cognitive and social impairments associated with the disease. In addition, growing evidence suggests that the increase in Kynurenic acid may begin early in life. For example, exposure to influenza A virus during development results in a transient increase in Kynurenic acid concentration that could disrupt normal brain development and lead to cognitive deficits later in life. Changes in Kynurenic acid may thus provide a link between developmental exposure to viruses and the increased risk of subsequently developing schizophrenia. To test this, we mimicked the effects of influenza A exposure by treating rats with kynurenine, the precursor of Kynurenic acid, on postnatal days 7-10. We observed a transient increase in both Kynurenic acid and quinolinic acid during treatment. When rats were subsequently behaviorally tested as adults, those previously treated with kynurenine exhibited decreased social behavior and locomotor activity. In contrast, attentional function and fear conditioning were not affected. Together with other recent findings, these data have several implications for understanding how viral-induced changes in tryptophan metabolism during development may contribute to schizophrenia-related symptoms later in life.
Kynurenic acid is a nutritional cue that enables behavioral plasticity.[Pubmed:25594177]
Cell. 2015 Jan 15;160(1-2):119-31.
The kynurenine pathway of tryptophan metabolism is involved in the pathogenesis of several brain diseases, but its physiological functions remain unclear. We report that Kynurenic acid, a metabolite in this pathway, functions as a regulator of food-dependent behavioral plasticity in C. elegans. The experience of fasting in C. elegans alters a variety of behaviors, including feeding rate, when food is encountered post-fast. Levels of neurally produced Kynurenic acid are depleted by fasting, leading to activation of NMDA-receptor-expressing interneurons and initiation of a neuropeptide-y-like signaling axis that promotes elevated feeding through enhanced serotonin release when animals re-encounter food. Upon refeeding, Kynurenic acid levels are eventually replenished, ending the elevated feeding period. Because tryptophan is an essential amino acid, these findings suggest that a physiological role of Kynurenic acid is in directly linking metabolism to activity of NMDA and serotonergic circuits, which regulate a broad range of behaviors and physiologies.
Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35.[Pubmed:16754668]
J Biol Chem. 2006 Aug 4;281(31):22021-8.
Local catabolism of the essential amino acid tryptophan is considered an important mechanism in regulating immunological and neurological responses. The kynurenine pathway is the main route for the non-protein metabolism of tryptophan. The intermediates of the kynurenine pathway are present at micromolar concentrations in blood and are regulated by inflammatory stimuli. Here we show that GPR35, a previously orphan G protein-coupled receptor, functions as a receptor for the kynurenine pathway intermediate Kynurenic acid. Kynurenic acid elicits calcium mobilization and inositol phosphate production in a GPR35-dependent manner in the presence of G(qi/o) chimeric G proteins. Kynurenic acid stimulates [35S]guanosine 5'-O-(3-thiotriphosphate) binding in GPR35-expressing cells, an effect abolished by pertussis toxin treatment. Kynurenic acid also induces the internalization of GPR35. Expression analysis indicates that GPR35 is predominantly detected in immune cells and the gastrointestinal tract. Furthermore, we show that Kynurenic acid inhibits lipopolysaccharide-induced tumor necrosis factor-alpha secretion in peripheral blood mononuclear cells. Our results suggest unexpected signaling functions for Kynurenic acid through GPR35 activation.
The "kynurenate test", a biochemical assay for putative cognition enhancers.[Pubmed:9336311]
J Pharmacol Exp Ther. 1997 Oct;283(1):82-90.
Some putative cognition enhancers (oxiracetam, aniracetam and D-cycloserine) were previously shown to prevent the Kynurenic acid antagonism of the N-methyl-D-aspartate (NMDA)-evoked norepinephrine (NE) release in rat hippocampal slices. This functional in vitro assay was further characterized in the present work. D-Serine, a glutamate coagonist at the NMDA receptor glycine site, concentration-dependently (EC50 approximately 0.1 microM) prevented the kynurenate (100 microM) block of the NMDA (100 microM)-evoked [3H]NE release. L-Serine was ineffective up to 10 microM. The gamma-aminobutyric acidB (GABA[B]) receptor antagonist CGP 36742, reported to improve cognitive performance, potently prevented the kynurenate antagonism. The activity of CGP 36742 (1 microM) appeared to be unaffected by 10 microM (-)-baclofen, a GABA(B) receptor agonist; furthermore, CGP 52432, a GABA(B) antagonist more potent than CGP 36742, but reportedly devoid of nootropic properties, was inactive in the "kynurenate test." The novel putative cognition enhancer CR2249, but not its enantiomer CR2361, also potently prevented the kynurenate antagonism. In contrast, linopirdine, nicotine and tacrine were inactive. In rat hippocampal synaptosomes glycine and D-cycloserine enhanced the NMDA-evoked [3H]NE release, whereas oxiracetam and CR2249 did not. These four compounds were all similarly effective in preventing kynurenate antagonism, both in slices and in synaptosomes. The NMDA potentiation caused by glycine (0.1-100 microM) was not affected by 100 microM oxiracetam, which suggested that drugs active in the "kynurenate test" may bind to sites different from the glycine site of the NMDA receptor. To conclude, the "kynurenate test" is an in vitro assay useful in the identification and characterization of putative cognition enhancers acting via NMDA receptors.


