CyperotundoneCAS# 3466-15-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3466-15-7 | SDF | Download SDF |
| PubChem ID | 12308615 | Appearance | Yellow liquid |
| Formula | C15H22O | M.Wt | 218.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
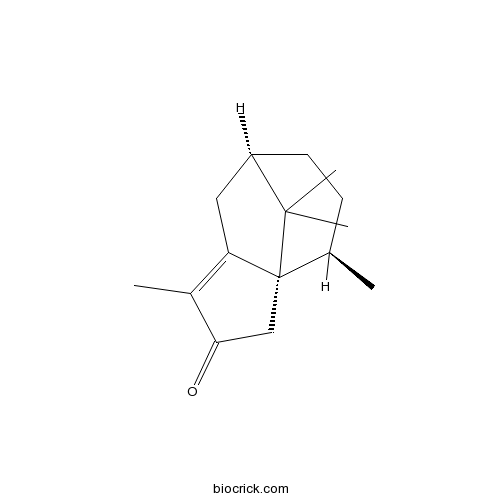
| Chemical Name | (1R,7R,10R)-4,10,11,11-tetramethyltricyclo[5.3.1.01,5]undec-4-en-3-one | ||
| SMILES | CC1CCC2CC3=C(C(=O)CC13C2(C)C)C | ||
| Standard InChIKey | GIGKXOAUYMWORB-OSQNNJELSA-N | ||
| Standard InChI | InChI=1S/C15H22O/c1-9-5-6-11-7-12-10(2)13(16)8-15(9,12)14(11,3)4/h9,11H,5-8H2,1-4H3/t9-,11-,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cyperotundone Dilution Calculator

Cyperotundone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.58 mL | 22.9001 mL | 45.8001 mL | 91.6003 mL | 114.5003 mL |
| 5 mM | 0.916 mL | 4.58 mL | 9.16 mL | 18.3201 mL | 22.9001 mL |
| 10 mM | 0.458 mL | 2.29 mL | 4.58 mL | 9.16 mL | 11.45 mL |
| 50 mM | 0.0916 mL | 0.458 mL | 0.916 mL | 1.832 mL | 2.29 mL |
| 100 mM | 0.0458 mL | 0.229 mL | 0.458 mL | 0.916 mL | 1.145 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Amoxicillin Sodium
Catalog No.:BCC4947
CAS No.:34642-77-8
- 1-Methoxycarbonyl-beta-carboline
Catalog No.:BCN5282
CAS No.:3464-66-2
- 2-Benzoyloxy-3-hydroxynortropane
Catalog No.:BCN1872
CAS No.:34622-25-8
- Maltohexaose
Catalog No.:BCN6710
CAS No.:34620-77-4
- Maltopentaose
Catalog No.:BCN8421
CAS No.:34620-76-3
- Maltotetraose
Catalog No.:BCN6709
CAS No.:34612-38-9
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
- AS601245
Catalog No.:BCC6464
CAS No.:345987-15-7
- Wilfornine A
Catalog No.:BCN3099
CAS No.:345954-00-9
- MM 11253
Catalog No.:BCC7782
CAS No.:345952-44-5
- Amicarbalide
Catalog No.:BCC8117
CAS No.:3459-96-9
- SUN-B 8155
Catalog No.:BCC7405
CAS No.:345893-91-6
- Dehydrodeguelin
Catalog No.:BCN4778
CAS No.:3466-23-7
- TCS 2510
Catalog No.:BCC7853
CAS No.:346673-06-1
- 1-Indanamine
Catalog No.:BCN2246
CAS No.:34698-41-4
- Incensole acetate
Catalog No.:BCN3830
CAS No.:34701-53-6
- Ferrostatin-1 (Fer-1)
Catalog No.:BCC2323
CAS No.:347174-05-4
- ITX3
Catalog No.:BCC6066
CAS No.:347323-96-0
- Sodium usnate
Catalog No.:BCN8376
CAS No.:34769-44-3
- Z-Asp(OBzl)-OH
Catalog No.:BCC2791
CAS No.:3479-47-8
- H-D-Met-OH
Catalog No.:BCC2997
CAS No.:348-67-4
- H-Tyr(Bzl)-OMe.HCl
Catalog No.:BCC3132
CAS No.:34805-17-9
- Boc-Met(O)-OH
Catalog No.:BCC3425
CAS No.:34805-21-5
- BAY 57-1293
Catalog No.:BCC4050
CAS No.:348086-71-5
Chemical investigation of Cyperus distans L. and inhibitory activity of scabequinone in seed germination and seedling growth bioassays.[Pubmed:24941231]
Nat Prod Res. 2014;28(23):2128-33.
Chemical investigation of the rhizomes of Cyperus distans (Cyperaceae) led to the identification of alpha-ciperone, Cyperotundone and scabequinone, besides other common constituents. Complete assignment of the (13)C NMR data of scabequinone is being published for the first time. The inhibitory effects of C. distans extracts and scabequinone on the seed germination and seedling growth of Mimosa pudica, Senna obtusifolia and Pueraria phaseoloides were evaluated. Seed germination inhibition bioassay revealed that S. obtusifolia (52-53%) was more sensitive to the hexane and the methanol extracts at 1% than M. pudica (0-10%). Scabequinone at 250 mg L(-)(1) displayed seed germination inhibitions more than 50% and radicle growth reduction of more than 35% of the test species S. obtusifolia and P. phaseoloides, while the hypocotyl growth of M. pudica was significantly affected (>50%) by the quinone at the same concentration. These results demonstrate that scabequinone contributes to the overall inhibitory activities of C. distans.
Acanthamoeba castellanii Neff: In vitro activity against the trophozoite stage of a natural sesquiterpene and a synthetic cobalt(II)-lapachol complex.[Pubmed:20045692]
Exp Parasitol. 2010 Sep;126(1):106-8.
In this study, the in vitro activities of a natural sesquiterpene, alpha-Cyperotundone, isolated from the root bark of Maytenus retusa and a cobalt(II)-complex of a natural occurring prenyl hydroxynaphthoquinone (lapachol) were evaluated against the trophozoite stage of Acanthamoeba castellanii Neff using a previously developed colorimetric 96-well microtiter plate assay, based on the oxido-reduction of Alamar Blue(R). The obtained activities showed that these two compounds were able to inhibit the in vitro growth of the amoebae at relatively low concentrations. Further identification of the molecular targets of these products and their effects on acanthamoebae should be determined to evaluate their possible therapeutic use.
Complete assignments of (1)H and (13)C NMR data for two new sesquiterpenes from Cyperus rotundus L.[Pubmed:19288546]
Magn Reson Chem. 2009 Jun;47(6):527-31.
Two new sesquiterpenes, epi-guaidiol A (1) and sugebiol (3), together with four known sesquiterpenes, guaidiol A(2), sugetriol triacetate (4), cyperenoic acid (5), and Cyperotundone (6) were isolated from the rhizomes of Cyperus rotundus L. Their structures were identified by MS and NMR experiments, and the complete assignments of (1)H and (13)C NMR data for two new sesquiterpenes were obtained by the aid of two-dimensional (2D) NMR techniques, including HSQC, HMBC, (1)H-(1)HCOSY and nuclear overhauser enhancement spectroscopy(NOESY).
The composition and antimicrobial activities of Cyperus conglomeratus, Desmos chinensis var. lawii and Cyathocalyx zeylanicus essential oils.[Pubmed:22799103]
Nat Prod Commun. 2012 May;7(5):663-6.
The essential oil compositions of the rhizomes of Cyperus conglomeratus (Cyperaceae) collected from Oman and the leaves of two Annonaceae plants, Desmos chinensis var. lawii and Cyathocalyx zeylanicus collected from India were studied by GC, GC-MS and 13C NMR spectroscopy. Twenty-six compounds, representing 84.4% of the oil were identified in C. conglomeratus, where eugenol (31.3%), alpha-cyperone (10.5%) and Cyperotundone (8.4%) were the major compounds. Twelve compounds, constituting 100%, were identified in D. lawii oil, of which benzyl benzoate (58.7%), beta-caryophyllene (23.2%), limonene (4.9%) and alpha-humulene (4.0%) were the major constituents. Thirty-two compounds, comprising 98.0%, were identified in C. zeylanicus oil, of which beta-caryophyllene (21.6%), alpha-pinene (20.4%) and E-beta-ocimene (11.8%) were the major components. The antibacterial and antifungal activities of the oils were tested against a panel of five bacterial and two fungal strains. The oils showed moderate activity against all the tested microbial strains. The minimum inhibitory concentrations of the oils were also determined.
Chemical profiling and biological properties of Neolitsea kedahense Gamble essential oils.[Pubmed:28278643]
Nat Prod Res. 2017 Dec;31(23):2793-2796.
Hydrodistillation of the fresh stem and leaf of Neolitsea kedahense Gamble, collected from Gunung Jerai, Malaysia followed by the GC-FID and GC-MS analysis revealed the detection of a total of 47 constituents of which 28 (86.4%) from the stem and 31 (96.4%) constituents from the leaf. delta-Cadinene (17.4%), 1-epi-cubenol (11.8%), Cyperotundone (9.0%), cis-cadin-4-en-7-ol (7.7%), tau-cadinol (7.1%) and alpha-cadinol (7.1%) were the principle constituents in the stem oil, whereas beta-caryophyllene (18.9%), bicyclogermacrene (18.6%) and trans-muurola-4(14),5-diene (9.8%) were the major constituents in the leaf oil. Among the identified constituents, three constituents namely 7-epi-alpha-selinene, junenol and cis-cadin-4-en-7-ol have not been found previously from Neolitsea oils. The stem and leaf oils were screened for their alpha-glucosidase inhibitory and antibacterial activities. Both oils displayed potential alpha-glucosidase inhibitory activity, while the stem oil possessed weak antibacterial activity against Bacillus subtilis.


