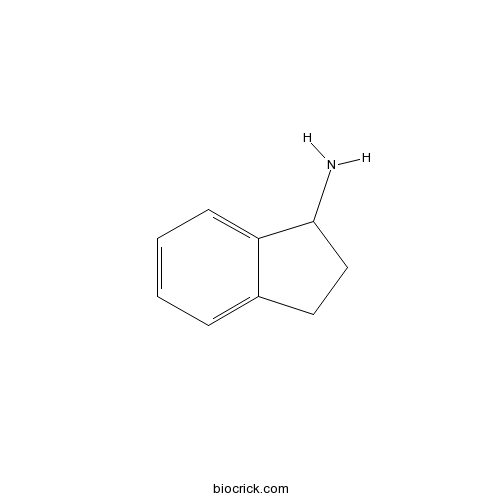
1-IndanamineCAS# 34698-41-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34698-41-4 | SDF | Download SDF |
| PubChem ID | 123445 | Appearance | Cryst. |
| Formula | C9H11N | M.Wt | 133.19 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,3-dihydro-1H-inden-1-amine | ||
| SMILES | C1CC2=CC=CC=C2C1N | ||
| Standard InChIKey | XJEVHMGJSYVQBQ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Tetrahedron, 1970, 26(1):107-118.Optically active amines—X: Optical rotatory dispersion and circular dichroism of the N-salicylidene derivatives of α- and β-phenylalkylamines—the absolute configurations of some phenylnorbornanes.[Reference: WebLink]ORD and CD measurements are reported for the N-salicylidene derivatives of a number of α and β-phenylalkylamines.
|

1-Indanamine Dilution Calculator

1-Indanamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.5081 mL | 37.5404 mL | 75.0807 mL | 150.1614 mL | 187.7018 mL |
| 5 mM | 1.5016 mL | 7.5081 mL | 15.0161 mL | 30.0323 mL | 37.5404 mL |
| 10 mM | 0.7508 mL | 3.754 mL | 7.5081 mL | 15.0161 mL | 18.7702 mL |
| 50 mM | 0.1502 mL | 0.7508 mL | 1.5016 mL | 3.0032 mL | 3.754 mL |
| 100 mM | 0.0751 mL | 0.3754 mL | 0.7508 mL | 1.5016 mL | 1.877 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TCS 2510
Catalog No.:BCC7853
CAS No.:346673-06-1
- Dehydrodeguelin
Catalog No.:BCN4778
CAS No.:3466-23-7
- Cyperotundone
Catalog No.:BCN8339
CAS No.:3466-15-7
- Amoxicillin Sodium
Catalog No.:BCC4947
CAS No.:34642-77-8
- 1-Methoxycarbonyl-beta-carboline
Catalog No.:BCN5282
CAS No.:3464-66-2
- 2-Benzoyloxy-3-hydroxynortropane
Catalog No.:BCN1872
CAS No.:34622-25-8
- Maltohexaose
Catalog No.:BCN6710
CAS No.:34620-77-4
- Maltopentaose
Catalog No.:BCN8421
CAS No.:34620-76-3
- Maltotetraose
Catalog No.:BCN6709
CAS No.:34612-38-9
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
- AS601245
Catalog No.:BCC6464
CAS No.:345987-15-7
- Wilfornine A
Catalog No.:BCN3099
CAS No.:345954-00-9
- Incensole acetate
Catalog No.:BCN3830
CAS No.:34701-53-6
- Ferrostatin-1 (Fer-1)
Catalog No.:BCC2323
CAS No.:347174-05-4
- ITX3
Catalog No.:BCC6066
CAS No.:347323-96-0
- Sodium usnate
Catalog No.:BCN8376
CAS No.:34769-44-3
- Z-Asp(OBzl)-OH
Catalog No.:BCC2791
CAS No.:3479-47-8
- H-D-Met-OH
Catalog No.:BCC2997
CAS No.:348-67-4
- H-Tyr(Bzl)-OMe.HCl
Catalog No.:BCC3132
CAS No.:34805-17-9
- Boc-Met(O)-OH
Catalog No.:BCC3425
CAS No.:34805-21-5
- BAY 57-1293
Catalog No.:BCC4050
CAS No.:348086-71-5
- 8-Acetonyldihydroavicine
Catalog No.:BCN3303
CAS No.:348098-59-9
- Marilactone
Catalog No.:BCN7363
CAS No.:34818-17-2
- 4'-Demethyleucomin
Catalog No.:BCN5283
CAS No.:34818-83-2
Synthesis and pharmacological evaluation of 3-(3,4-dichlorophenyl)-1-indanamine derivatives as nonselective ligands for biogenic amine transporters.[Pubmed:15115403]
J Med Chem. 2004 May 6;47(10):2624-34.
In our efforts toward developing a nonselective ligand that would block the effects of stimulants such as methamphetamine at dopamine (DA), serotonin (5-HT), and norepinephrine (NE) transporters, we synthesized a series of 3-(3,4-dichlorophenyl)-1-Indanamine derivatives. Two of the examined higher affinity compounds had a phenolic hydroxyl group enabling preparation of a medium to long chain carboxylic acid ester that might eventually be useful for a long-acting depot formulation. The in vitro data indicated that (-)-(1R,3S)-trans-3-(3,4-dichlorophenyl)-6-hydroxy-N-methyl-1-Indanamine ((-)-(1R,3S)-11) displays high-affinity binding and potent inhibition of uptake at all three biogenic amine transporters. In vivo microdialysis experiments demonstrated that intravenous administration of (-)-(1R,3S)-11 to rats elevated extracellular DA and 5-HT in the nucleus accumbens in a dose-dependent manner. Pretreating rats with 0.5 mg/kg (-)-(1R,3S)-11 elevated extracellular DA and 5-HT by approximately 150% and reduced methamphetamine-induced neurotransmitter release by about 50%. Ex vivo autoradiography, however, demonstrated that iv administration of (-)-(1R,3S)-11 produced a dose-dependent, persistent occupation of 5-HT transporter binding sites but not DA transporter sites.
Slow-onset, long-duration 3-(3',4'-dichlorophenyl)-1-indanamine monoamine reuptake blockers as potential medications to treat cocaine abuse.[Pubmed:11150168]
J Med Chem. 2000 Dec 28;43(26):4981-92.
A series of 3-(3',4'-dichlorophenyl)-1-Indanamine monoamine reuptake blockers have been synthesized in an effort to develop a compound that could be used as a maintenance therapy to treat cocaine abuse. Since the effects of cocaine on dopamine (DA) and serotonin (5HT) transporters are important components of its pharmacological activity, the focus was on nonselective inhibitors of monoamine transport. To reduce or eliminate the abuse potential of a DA reuptake blocker, the compounds were designed to be slow-onset, long-duration prodrugs whose N-demethylated metabolites would have increased activity over the parent compound with the ideal being a parent compound that has little or no activity. To achieve this, pairs of compounds with different groups on the amine nitrogen and with and without an additional N-methyl group were synthesized. All of the synthesized compounds were screened for binding and reuptake at the cloned human DA, 5HT, and norepinephrine (NE) transporters. As previously found, trans isomers are nonselective blockers of DA, 5HT, and NE reuptake, cis isomers with small N-alkyl groups are selective blockers of 5HT reuptake, and tertiary amines of the trans compounds are less potent than the corresponding N-demethylated secondary amines as blockers of DA reuptake. Larger N-alkyl groups in both the trans and cis series were found to reduce activity for the 5HT and NE transporters with less effect at DA transporters. Selected trans compounds were also screened for locomotor activity in mice and generalization to a cocaine-like profile in rats. With intraperitoneal administration, all of the trans isomers showed a slow onset of at least 20 min and an extremely long duration of action in the locomotor assays. Several of the trans compounds also fully generalized to a cocaine-like pharmacological profile. An initial lead compound, the N,N-dimethyl analogue trans-1b, was resolved into chirally pure enantiomers. Surprisingly, both enantiomers were found to have significant affinity for the DA transporter and to cause locomotor activation. This is in contrast to the N-methyl compound in which only the (+)-enantiomer had significant activity. The absolute configuration of the more active enantiomer was determined by X-ray crystallography to be 3R,1S.


