Stellera chamaejasme
Stellera chamaejasme
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Stellera chamaejasme
- Cat.No. Product Name CAS Number COA
-
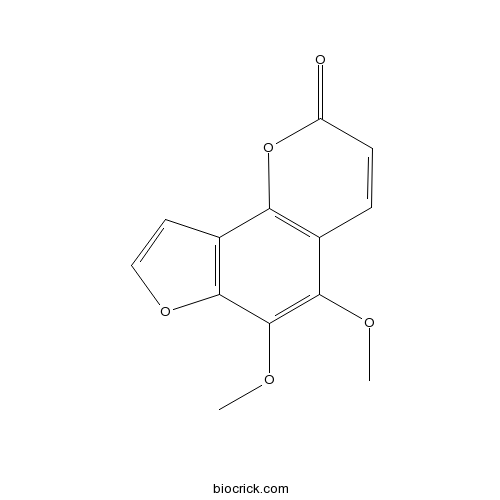
BCN6168
Pimpinellin131-12-4
Instructions
-
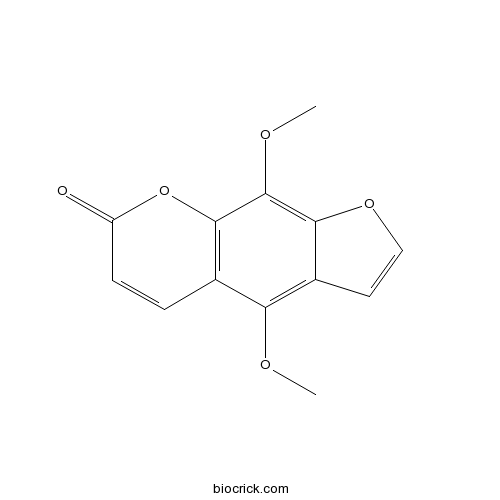
BCN5568
Isopimpinellin482-27-9
Instructions
-
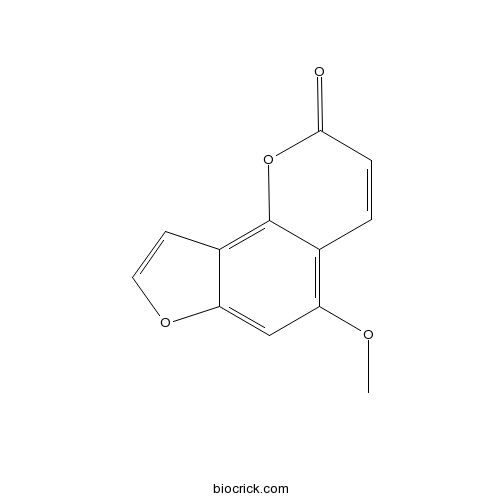
BCN2377
Isobergapten482-48-4
Instructions
-
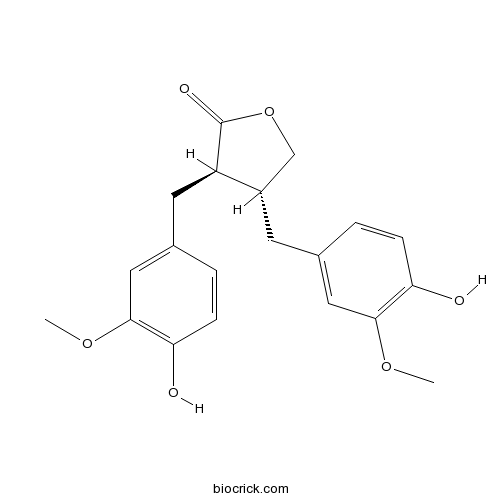
BCN5789
Matairesinol580-72-3
Instructions
-
BCN4477
Umbelliferone93-35-6
Instructions

[A new lignan from rhizome of Stellera chamaejasme].[Pubmed: 29751712]
To investigate the chemical compounds from the rhizome of Stellera chamaejasme, nine lignans, including stellerachamin A (1), 8-hydroxypluviatolide (2), wikstromol (3), pinoresinol (4), matairesinol (5), dextrobursehernin (6), hinokinin(7), (-)-glaberide I (8) and (-) medioresinol (9) were isolated by various chromatographic methods. Their structures were extensively determined on basis of MS and NMR spectroscopic data analysis. Among them, compound 1 was a new lignan, and compounds 2 and 7 were isolated from Thymelaeaceae for the first time.
A new flavonoid from Stellera chamaejasme L., stechamone, alleviated 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in a murine model.[Pubmed: 29653408]
None
A UPLC-MS/MS method for simultaneous determination of five flavonoids from Stellera chamaejasme L. in rat plasma and its application to a pharmacokinetic study.[Pubmed: 29328498]
None
Simultaneous determination of isochamaejasmin, neochamaejasmin A and aphnoretinin rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study of Stellera chamaejasme L. extract.[Pubmed: 29235122]
Isochamaejasmin, neochamaejasmin A and daphnoretin derived from Stellera chamaejasme L. are important because of their reported anticancer properties. In this study, a sensitive UPLC-MS/MS method for the determination of isochamaejasmin, neochamaejasmin A and daphnoretin in rat plasma was developed. The analyte and IS were separated on an Acquity UPLC HSS T3 column (100 × 2.1 mm, 1.8 μm) using gradient elution with the mobile phase of aqueous solution (methanol-water, 1:99, v/v, containing 1 mm formic acid) and organic solution (methanol-water, 99:1, v/v, containing 1 mm formic acid) at a flow rate of 0.3 mL/min. Multiple reaction monitoring mode with negative electrospray ionization interface was carried out to detect the components. The method was validated in terms of specificity, linearity, accuracy, precision, stability, etc. Excellent linear behavior was observed over the certain concentration ranges with the correlation coefficient values >0.99. Intra- and inter-day precisions (RSD) were <6.7% and accuracy (RE) ranged from -7.0 to 12.0%. The validated method was successfully applied to investigate the pharmacokinetics of three chemical ingredients after oral administration of S. chamaejasme L. extract to rats.
Stellera chamaejasme and its constituents induce cutaneous wound healing and anti-inflammatory activities.[Pubmed: 28220834]
None
Chamaejasmine induces apoptosis in HeLa cells through the PI3K/Akt signaling pathway.[Pubmed: 27557139]
Chamaejasmine is one of the major bioactive components of Stellera chamaejasme L, which is a Chinese traditional herbal medicine that has been used widely in the treatment of cancer. The aim of this study is to investigate the potential effect of chamaejasmine on cervical cancer cells and elucidate the underlying mechanisms of action. We first examined the antitumor activity of chamaejasmine both in vitro and in vivo. In the following experiments with HeLa cells, cell apoptosis and ultrastructure changes were assessed by flow cytometry and transmission electron microscopy, respectively. The effects of chamaejasmine on reactive oxygen species production and mitochondrial membrane potential (Δψm) were examined using 2',7'-dichlorohydrofluorescein and rhodamine-123 staining. The mRNA and protein levels of apoptosis-related proteins were detected by real-time PCR and western blot. The activity of caspases 3, 8, and 9 was measured using the corresponding assay kit. The effect of chamaejasmine on the phosphoinositide 3-kinase (PI3K)/Akt pathway was evaluated by luciferase assay and western blot, and further confirmed in Akt overexpressing HeLa cells. We found that chamaejasmine has potent antitumor effects on cervical cancer cell lines both in vitro and in vivo. Mechanistic studies showed that chamaejasmine could induce apoptosis in HeLa cells, and this apoptosis-inducing effect may be mediated through the suppression of PI3K/Akt signaling cascades. These findings not only indicate the therapeutic potential of chamaejasmine for cervical cancer, but provide valuable insight into its mechanism of action.


