dehydrotomatineCAS# 157604-98-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 157604-98-3 | SDF | Download SDF |
| PubChem ID | 122391230.0 | Appearance | Powder |
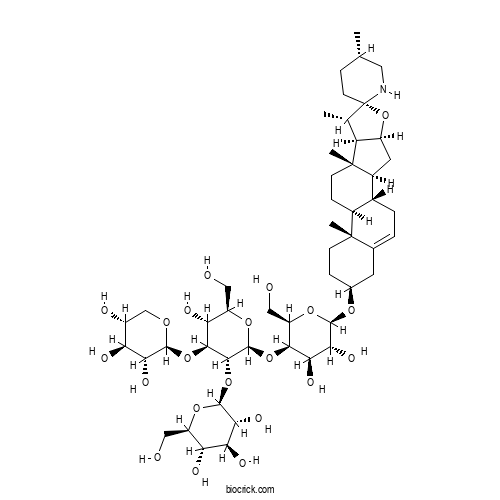
| Formula | C50H81NO21 | M.Wt | 1032.18 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[(2S,3R,4S,5R,6R)-2-[(2R,3R,4R,5R,6R)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(1S,2S,4S,5'S,6S,7S,8R,9S,12S,13R,16S)-5',7,9,13-tetramethylspiro[5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icos-18-ene-6,2'-piperidine]-16-yl]oxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)-4-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC1CCC2(C(C3C(O2)CC4C3(CCC5C4CC=C6C5(CCC(C6)OC7C(C(C(C(O7)CO)OC8C(C(C(C(O8)CO)O)OC9C(C(C(CO9)O)O)O)OC2C(C(C(C(O2)CO)O)O)O)O)O)C)C)C)NC1 | ||
| Standard InChIKey | BYMOGFTUZUEFHY-SIUCFGLGSA-N | ||
| Standard InChI | InChI=1S/C50H81NO21/c1-20-7-12-50(51-15-20)21(2)32-28(72-50)14-26-24-6-5-22-13-23(8-10-48(22,3)25(24)9-11-49(26,32)4)65-45-40(63)37(60)41(31(18-54)68-45)69-47-43(71-46-39(62)36(59)34(57)29(16-52)66-46)42(35(58)30(17-53)67-47)70-44-38(61)33(56)27(55)19-64-44/h5,20-21,23-47,51-63H,6-19H2,1-4H3/t20-,21-,23-,24+,25-,26-,27+,28-,29+,30+,31+,32-,33-,34+,35+,36-,37+,38+,39+,40+,41-,42-,43+,44-,45+,46-,47-,48-,49-,50-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

dehydrotomatine Dilution Calculator

dehydrotomatine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.9688 mL | 4.8441 mL | 9.6882 mL | 19.3765 mL | 24.2206 mL |
| 5 mM | 0.1938 mL | 0.9688 mL | 1.9376 mL | 3.8753 mL | 4.8441 mL |
| 10 mM | 0.0969 mL | 0.4844 mL | 0.9688 mL | 1.9376 mL | 2.4221 mL |
| 50 mM | 0.0194 mL | 0.0969 mL | 0.1938 mL | 0.3875 mL | 0.4844 mL |
| 100 mM | 0.0097 mL | 0.0484 mL | 0.0969 mL | 0.1938 mL | 0.2422 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Debenzoylpaeoniflorgenin
Catalog No.:BCX1516
CAS No.:1429403-79-1
- Paeoniflorgenin
Catalog No.:BCX1515
CAS No.:697300-41-7
- Synigrin
Catalog No.:BCX1514
CAS No.:534-69-0
- Rhetsinine
Catalog No.:BCX1513
CAS No.:526-43-2
- Smilagenin acetate
Catalog No.:BCX1512
CAS No.:4947-75-5
- Lycoramine Hydrobromide
Catalog No.:BCX1511
CAS No.:89505-76-0
- Isocoptisine acetate
Catalog No.:BCX1510
CAS No.:30426-66-5
- Isoemetine hydrobromide
Catalog No.:BCX1509
CAS No.:21026-77-7
- 4'-Hydroxyflavone
Catalog No.:BCX1508
CAS No.:4143-63-9
- Sarsasapogenin acetate
Catalog No.:BCX1507
CAS No.:35319-91-6
- Fulvotomentoside A
Catalog No.:BCX1506
CAS No.:150107-44-1
- Tetrahydrodehydrodiconiferyl alcohol
Catalog No.:BCX1505
CAS No.:5234-70-8
- Norfuronol
Catalog No.:BCX1518
CAS No.:19322-27-1
- Resveratrol-4'-O-(6"-galloyl)-β-D-glucopyranoside
Catalog No.:BCX1519
CAS No.:928340-97-0
- Prosaikogenin A
Catalog No.:BCX1520
CAS No.:99365-21-6
- Nortanshinone
Catalog No.:BCX1521
CAS No.:97399-70-7
- Acetylseneciphylline N-oxide
Catalog No.:BCX1522
CAS No.:123844-00-8
- Dahurinol
Catalog No.:BCX1523
CAS No.:38908-87-1
- Luteolin 7-sulfate
Catalog No.:BCX1524
CAS No.:56857-57-9
- Bitalgenin
Catalog No.:BCX1525
CAS No.:2192-25-8
- (±)-Dihydrokaempferol
Catalog No.:BCX1526
CAS No.:104486-98-8
- Isoescin Ie
Catalog No.:BCX1527
CAS No.:1613506-28-7
- Dihydroactindiolide
Catalog No.:BCX1528
CAS No.:15356-74-8
- 4-Vinylphenol
Catalog No.:BCX1529
CAS No.:2628-17-3
Physiological functions, pharmacological aspects and nutritional importance of green tomato- a future food.[Pubmed:37267154]
Crit Rev Food Sci Nutr. 2023 Jun 2:1-29.
Green tomatoes contain significant levels of steroidal glycoalkoids (SGA) such as alpha-tomatine and green pigment chlorophyll. Tomatine is an admixture of two glycoalkoids; alpha tomatine and dehydrotomatine reported various health beneficial biological activities. Moreover, a hydrolyzed product of tomatine also contributes to age-related atrophy, and muscle weakness and helps the elderly recover from illness and injuries related to age. However, there is a lack of evidence regarding the absorption of tomatine in the human body concerning proposed biological activity, which should be an area of interest in the future. Once, the absorption study is established compounds concentrated in green tomatoes are potentially involved as protective compounds for several diseases and also used for functional food. To facilitate the use of green tomatoes in food processing, this comprehensive review provides data on the nutritional value of green tomatoes, with emphasis on the evolution of the physiological chemistry, analytical, medicinal, and pharmacological effects of the alpha-tomatine and chlorophyll in an experimental model. The broad aim of this review is to evaluate the health benefits of green tomatoes in addition to their nutritional value and to study the several features of the role of alpha-tomatine and chlorophyll in human health.
Isomers of the Tomato Glycoalkaloids alpha-Tomatine and Dehydrotomatine: Relationship to Health Benefits.[Pubmed:37110854]
Molecules. 2023 Apr 21;28(8):3621.
High-performance liquid chromatography (HPLC) analysis of three commercial tomatine samples and another isolated from green tomatoes revealed the presence of two small peaks in addition to those associated with the glycoalkaloids dehydrotomatine and alpha-tomatine. The present study investigated the possible structures of the compounds associated with the two small peaks using HPLC-mass spectrophotometric (MS) methods. Although the two peaks elute much earlier on chromatographic columns than the elution times of the known tomato glycoalkaloids dehydrotomatine and alpha-tomatine, isolation of the two compounds by preparative chromatography and subsequent analysis by MS shows the two compounds have identical molecular weights, tetrasaccharide side chains, and MS and MS/MS fragmentation patterns to dehydrotomatine and alpha-tomatine. We suggest the two isolated compounds are isomeric forms of dehydrotomatine and alpha-tomatine. The analytical data indicate that widely used commercial tomatine preparations and those extracted from green tomatoes and tomato leaves consist of a mixture of alpha-tomatine, dehydrotomatine, an alpha-tomatine isomer, and a dehydrotomatine isomer in an approximate ratio of 81:15:4:1, respectively. The significance of the reported health benefits of tomatine and tomatidine is mentioned.
Evidence for Cardiac Glycosides in Foliage of Colorado Potato Beetle-Resistant Solanum okadae.[Pubmed:36351172]
J Agric Food Chem. 2022 Nov 23;70(46):14613-14621.
Leptinotarsa decemlineata, the Colorado potato beetle (CPB), is a herbivore that primarily feeds on Solanum foliage and is a global pest of the potato agricultural industry. Potato breeding through cross-hybridization with CPB-resistant wild relatives is used for genetic improvement. The wild species Solanum okadae was demonstrated to deter CPB feeding in choice and no choice feeding assays. Liquid chromatography-mass spectrometry (LC-MS) was used for comparative metabolite profiling between S. okadae and CPB-susceptible domesticated potato variety, Solanum tuberosum cv. Shepody. Major foliar metabolites detected were steroidal glycoalkaloids (SGAs) with tomatine and dehydrotomatine produced in S. okadae and solanine and chaconine in S. tuberosum cv. Shepody. Cardiac glycosides were also detected in the foliar metabolite profile of S. okadae but not S. tuberosum cv. Shepody. This class of plant compounds have known insecticidal activity through inhibition of animal Na(+)/K(+) ATPase. Thin-layer chromatography (TLC) separation of foliar extracts also provided evidence for cardiac glycosides in S. okadae. Cardiac glycosides are known inhibitors of Na(+)/K(+) ATPase, and foliar extracts from S. okadae (OKA15), but not S. tuberosum cv. Shepody, were able to inhibit the Na(+)/K(+) ATPase of CPB. These findings suggest a novel mechanism of plant resistance against CPB involving production of cardiac glycosides in S. okadae.
The basic helix-loop-helix transcription factors MYC1 and MYC2 have a dual role in the regulation of constitutive and stress-inducible specialized metabolism in tomato.[Pubmed:35838067]
New Phytol. 2022 Nov;236(3):911-928.
Plants produce specialized metabolites to protect themselves from biotic enemies. Members of the Solanaceae family accumulate phenylpropanoid-polyamine conjugates (PPCs) in response to attackers while also maintaining a chemical barrier of steroidal glycoalkaloids (SGAs). Across the plant kingdom, biosynthesis of such defense compounds is promoted by jasmonate signaling in which clade IIIe basic helix-loop-helix (bHLH) transcription factors play a central role. By characterizing hairy root mutants obtained through Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-CRISPR associated protein 9 (CRISPR-Cas9) genome editing, we show that the tomato clade IIIe bHLH transcription factors, MYC1 and MYC2, redundantly control jasmonate-inducible PPC and SGA production, and are also essential for constitutive SGA biosynthesis. Double myc1 myc2 loss-of-function tomato hairy roots displayed suppressed constitutive expression of SGA biosynthesis genes, and severely reduced levels of the main tomato SGAs alpha-tomatine and dehydrotomatine. In contrast, basal expression of genes involved in PPC biosynthesis was not affected. CRISPR-Cas9(VQR) genome editing of a specific cis-regulatory element, targeted by MYC1/2, in the promoter of a SGA precursor biosynthesis gene led to decreased constitutive expression of this gene, but did not affect its jasmonate inducibility. Our results demonstrate that clade IIIe bHLH transcriptional regulators have evolved under the control of distinct regulatory cues to specifically steer constitutive and stress-inducible specialized metabolism.
Combined Experimental and Multivariate Model Approaches for Glycoalkaloid Quantification in Tomatoes.[Pubmed:34063803]
Molecules. 2021 May 21;26(11):3068.
The intake of tomato glycoalkaloids can exert beneficial effects on human health. For this reason, methods for a rapid quantification of these compounds are required. Most of the methods for alpha-tomatine and dehydrotomatine quantification are based on chromatographic techniques. However, these techniques require complex and time-consuming sample pre-treatments. In this work, HPLC-ESI-QqQ-MS/MS was used as reference method. Subsequently, multiple linear regression (MLR) and partial least squares regression (PLSR) were employed to create two calibration models for the prediction of the tomatine content from thermogravimetric (TGA) and attenuated total reflectance (ATR) infrared spectroscopy (IR) analyses. These two fast techniques were proven to be suitable and effective in alkaloid quantification (R(2) = 0.998 and 0.840, respectively), achieving low errors (0.11 and 0.27%, respectively) with the reference technique.
Supramolecular Assembly of Hybrid Pt(II) Porphyrin/Tomatine Analogues with Different Nanostructures and Cytotoxic Activities.[Pubmed:34056476]
ACS Omega. 2021 May 4;6(20):13284-13292.
A simple strategy for synthesizing supramolecular hybrids was developed for the preparation of bioavailable nanohybrid photosensitizers by assembling visible-light-sensitive Pt(II) meso-tetrakis(4-carboxyphenyl)porphyrinporphyrin (PtTCPP)/tomatine analogues. The hybrids were self-assembled into nanofibrous or nanosheet structures approximately 3-5 nm thick and several micrometers wide. alpha-Tomatine generated a unique fibrous vesicle nanostructure based on intermolecular interactions, while dehydrotomatine generated nanosheet structures. Nanoassembly of these fibrous vesicles and sheets directly affected the properties of the light-responsive photosensitizer for tumor photodynamic therapy (PDT), depending on the nanostructure of the hybrid PtTCPP/tomatine analogues. The cytotoxicity of PtTCPP to cancer cells under photoirradiation was significantly enhanced by a tomatine assembly with a fibrous vesicle nanostructure, attributable to increased incorporation of the drug into cells.
Isolate-Specific Effect of Entomopathogenic Endophytic Fungi on Population Growth of Two-Spotted Spider Mite (Tetranychus urticae Koch) and Levels of Steroidal Glycoalkaloids in Tomato.[Pubmed:33740175]
J Chem Ecol. 2021 May;47(4-5):476-488.
Entomopathogenic fungi (EPF) can be experimentally established in several plant species as endophytes. Ecological effects of EPF inoculations on plant growth and plant-herbivore interactions have been demonstrated, potentially by altering plant physiological responses. However, the role of these responses in plant-fungus-herbivore tripartite interactions has not been well elucidated. Steroidal glycoalkaloids (SGAs) are plant specialized metabolites with bioactive properties against arthropod herbivores. Here, the effects of seed treatments by three EPF isolates, representing Beauveria bassiana, Metarhizium brunneum, and M. robertsii, on population growth of two-spotted spider mites (Tetranychus urticae Koch) were evaluated on tomato (Solanum lycopersicum). The levels of two SGAs, alpha-tomatine and dehydrotomatine, were determined in tomato leaves by LC-MS with and without T. urticae infestations after EPF inoculations. Interestingly, the population growth of T. urticae was significantly highest with M. brunneum and lowest with M. robertsii and B. bassiana at 15 days after infestation. Overall there was a significant negative correlation between SGAs content and the number of T. urticae. The levels of SGAs were significantly induced by T. urticae presence in all treatments, while only M. robertsii showed significantly higher levels of SGAs than M. brunneum and control in one of two experiments. Contrastingly, the effects on SGAs accumulation and population growth of T. urticae did not directly correlate with EPF endophytic colonization patterns of the inoculated plants. This study suggests a link between ecological effects and physiological responses mediated by EPF inoculations and T. urticae infestation with potential implications for plant protection.
Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. var. "Camone" Tomato in Spontaneously Hypertensive Rats.[Pubmed:32824747]
Molecules. 2020 Aug 18;25(16):3758.
Blood pressure control in hypertensive subjects calls for changes in lifestyle, especially diet. Tomato is widely consumed and rich in healthy components (i.e., carotenoids, vitamins and polyphenols). The aim of this study was to evaluate the chemical composition and antihypertensive effects of locular gel reconstituted in serum of green tomatoes of "Camone" variety. Tomato serum and locular gel were chemically characterised. The antihypertensive effects of the locular gel in serum, pure tomatine, and captopril, administered by oral gavage, were investigated for 4 weeks in male spontaneously hypertensive and normotensive rats. Systolic blood pressure and heart rate were monitored using the tail cuff method. Body and heart weight, serum glucose, triglycerides and inflammatory cytokines, aorta thickness and liver metabolising activity were also assessed. Locular gel and serum showed good tomatine and polyphenols content. Significant reductions in blood pressure and heart rate, as well as in inflammatory blood cytokines and aorta thickness, were observed in spontaneously hypertensive rats treated both with locular gel in serum and captopril. No significant effects were observed in normotensive rats. Green tomatoes locular gel and serum, usually discarded during tomato industrial processing, are rich in bioactive compounds (i.e., chlorogenic acid, caffeic acid and rutin, as well as the glycoalkaloids, alpha-tomatine and dehydrotomatine) that can lower in vivo blood pressure towards healthier values, as observed in spontaneously hypertensive rats.
Tomatine Displays Antitumor Potential in In Vitro Models of Metastatic Melanoma.[Pubmed:32718103]
Int J Mol Sci. 2020 Jul 23;21(15):5243.
There is a growing interest in the cytotoxic effects of bioactive glycoalkaloids, such as alpha-tomatine on tumor cells. Here, for the first time, we determine the antitumor potential of tomatine, a mixture of alpha-tomatine and dehydrotomatine, in metastatic melanoma (MM) cell lines harboring different BRAF and MC1R variants. We performed cytotoxicity experiments and annexin-V/propidium iodide staining to assess the apoptotic/necrotic status of the cells. ER stress and autophagy markers were revealed by Western Blot, whereas antiangiogenic and vascular-disrupting effects were evaluated through a capillary tube formation assay on matrigel and by ELISA kit for VEGF release determination. Cell invasion was determined by a Boyden chamber matrigel assay. Tomatine reduced 50% of cell viability and induced a concentration-dependent increase of apoptotic cells in the range of 0.5-1 muM in terms of alpha-tomatine. The extent of apoptosis was more than two-fold higher in (V600)BRAF-D184H/D184H MC1R cells than in BRAF wild-type cells and (V600)BRAF-MC1R wild-type cell lines. Additionally, tomatine increased the LC3I/II autophagy marker, p-eIF2alpha, and p-Erk1/2 levels in BRAF wild-type cells. Notably, tomatine strongly reduced cell invasion and melanoma-dependent angiogenesis by reducing VEGF release and tumor-stimulating effects on capillary tube formation. Collectively, our findings support tomatine as a potential antitumor agent in MM.
Characterization of steroid 5alpha-reductase involved in alpha-tomatine biosynthesis in tomatoes.[Pubmed:31983879]
Plant Biotechnol (Tokyo). 2019 Dec 25;36(4):253-263.
alpha-tomatine and dehydrotomatine are steroidal glycoalkaloids (SGAs) that accumulate in the mature green fruits, leaves, and flowers of tomatoes (Solanum lycopersicum) and function as defensive compounds against pathogens and predators. The aglycones of alpha-tomatine and dehydrotomatine are tomatidine and dehydrotomatidine (5,6-dehydrogenated tomatidine), and tomatidine is derived from dehydrotomatidine via four reaction steps: C3 oxidation, isomerization, C5alpha reduction, and C3 reduction. Our previous studies (Lee et al. 2019) revealed that Sl3betaHSD is involved in the three reactions except for C5alpha reduction, and in the present study, we aimed to elucidate the gene responsible for the C5alpha reduction step in the conversion of dehydrotomatidine to tomatidine. We characterized the two genes, SlS5alphaR1 and SlS5alphaR2, which show high homology with DET2, a brassinosteroid 5alpha reductase of Arabidopsis thaliana. The expression pattern of SlS5alphaR2 is similar to those of SGA biosynthetic genes, while SlS5alphaR1 is ubiquitously expressed, suggesting the involvement of SlS5alphaR2 in SGA biosynthesis. Biochemical analysis of the recombinant proteins revealed that both of SlS5alphaR1 and SlS5alphaR2 catalyze the reduction of tomatid-4-en-3-one at C5alpha to yield tomatid-3-one. Then, SlS5alphaR1- or SlS5alphaR2-knockout hairy roots were constructed using CRISPR/Cas9 mediated genome editing. In the SlS5alphaR2-knockout hairy roots, the alpha-tomatine level was significantly decreased and dehydrotomatine was accumulated. On the other hand, no change in the amount of alpha-tomatine was observed in the SlS5alphaR1-knockout hairy root. These results indicate that SlS5alphaR2 is responsible for the C5alpha reduction in alpha-tomatine biosynthesis and that SlS5alphaR1 does not significantly contribute to alpha-tomatine biosynthesis.
Identification of a 3beta-Hydroxysteroid Dehydrogenase/ 3-Ketosteroid Reductase Involved in alpha-Tomatine Biosynthesis in Tomato.[Pubmed:30892648]
Plant Cell Physiol. 2019 Jun 1;60(6):1304-1315.
alpha-Tomatine and dehydrotomatine are major steroidal glycoalkaloids (SGAs) that accumulate in the mature green fruits, leaves and flowers of tomato (Solanum lycopersicum), and function as defensive compounds against bacteria, fungi, insects and animals. The aglycone of dehydrotomatine is dehydrotomatidine (5,6-dehydrogenated tomatidine, having the Delta5,6 double bond; the dehydro-type). The aglycone of alpha-tomatine is tomatidine (having a single bond between C5 and C6; the dihydro-type), which is believed to be derived from dehydrotomatidine via four reaction steps: C3 oxidation, isomerization, C5 reduction and C3 reduction; however, these conversion processes remain uncharacterized. In the present study, we demonstrate that a short-chain alcohol dehydrogenase/reductase designated Sl3betaHSD is involved in the conversion of dehydrotomatidine to tomatidine in tomato. Sl3betaHSD1 expression was observed to be high in the flowers, leaves and mature green fruits of tomato, in which high amounts of alpha-tomatine are accumulated. Biochemical analysis of the recombinant Sl3betaHSD1 protein revealed that Sl3betaHSD1 catalyzes the C3 oxidation of dehydrotomatidine to form tomatid-4-en-3-one and also catalyzes the NADH-dependent C3 reduction of a 3-ketosteroid (tomatid-3-one) to form tomatidine. Furthermore, during co-incubation of Sl3betaHSD1 with SlS5alphaR1 (steroid 5alpha-reductase) the four reaction steps converting dehydrotomatidine to tomatidine were completed. Sl3betaHSD1-silenced transgenic tomato plants accumulated dehydrotomatine, with corresponding decreases in alpha-tomatine content. Furthermore, the constitutive expression of Sl3betaHSD1 in potato hairy roots resulted in the conversion of potato SGAs to the dihydro-type SGAs. These results demonstrate that Sl3betaHSD1 is a key enzyme involved in the conversion processes from dehydrotomatidine to tomatidine in alpha-tomatine biosynthesis.
Fragmentation study of major spirosolane-type glycoalkaloids by collision-induced dissociation linear ion trap and infrared multiphoton dissociation Fourier transform ion cyclotron resonance mass spectrometry.[Pubmed:27593526]
Rapid Commun Mass Spectrom. 2016 Nov 30;30(22):2395-2406.
RATIONALE: Glycoalkaloids play a key role in the plant protection system against phytopathogens including fungi, viruses, bacteria, insects and worms. They can be toxic to humans if consumed in high concentrations causing gastrointestinal disturbances. METHODS: The structural characterization of the major spirosolane glycoalkaloids, solasonine, solamargine, alpha-tomatine and dehydrotomatine, were investigated by positive electrospray ionization (ESI) coupled with a hybrid linear ion trap (LIT) and Fourier transform ion cyclotron resonance (FTICR) mass spectrometer. Tandem mass spectrometric analysis of spirosolane glycoalkaloids was performed by both collision-induced dissociation (CID) within the LIT and infrared multiphoton dissociation (IRMPD) in conjunction with the FTICR cell. RESULTS: Several common product ions were observed, generated by losses of the sugar moiety or aglycone fragmentation in the B- or E-ring, that can provide information on the accurate mass of aglycone and the primary sequence and branching of the oligosaccharide chains. Thanks to the multistage CID it was possible to understand the fragmentation pathways and thanks to the high resolution of IRMPD-FTICR the elemental compositions of product ions were obtained. CONCLUSIONS: Because the investigated tandem mass spectra data were acquired with high mass accuracy, unambiguous interpretation and determination of the chemical compositions for the majority of detected fragment ions were feasible. From these data, generalized fragmentation pathways were proposed, providing guidance for the characterization of unknown glycoalkaloids in plants. Copyright (c) John Wiley & Sons, Ltd.
High-performance liquid chromatography LTQ-Orbitrap mass spectrometry method for tomatidine and non-target metabolites quantification in organic and normal tomatoes.[Pubmed:25156359]
Int J Food Sci Nutr. 2014 Dec;65(8):942-7.
Tomatoes, members of the Solanaceae plant family, produce biologically active secondary metabolites, including glycoalkaloids and aglycons, which may have both adverse and beneficial biological effects. A new liquid chromatography method that utilized LTQ-Orbitrap MS was developed for the analysis of tomatidine, the main aglycon in tomatoes. Recoveries of tomatidine were >98.3% with the relative standard deviations (RSDs) below 6.1%. The limit of detection (LODs) was 0.0003 mg kg(-1). The limit of quantitation (LOQs) is 0.001 mg kg(-1). The linear range was between with 0.0025 and 1 mg kg(-1) with an excellent correlation coefficient (R(2)) equal to 0.9990. Various tomato samples were analyzed and the level of tomatidine in the 11 samples analysed was higher in normal respect to organic tomatoes. The capability of the set-up Full Scan LTQ-Orbitrap MS method allowed us to quantified two non-target analytes. The m/z 1032 was identified as dehydrotomatine, confirmed through accurate mass studies (mass error in ppm equal to 1.5017) meanwhile m/z 902 as (Glc)2-Gal-Tomatidine (beta1-Tomatine) (mass error in ppm equal to 2.0719).
Steroidal glycoalkaloid profiling and structures of glycoalkaloids in wild tomato fruit.[Pubmed:23941899]
Phytochemistry. 2013 Nov;95:145-57.
Steroidal glycoalkaloids (SGAs) constitute one of the main groups of secondary metabolites in tomato fruit. However, the detailed composition of SGAs other than alpha-tomatine, dehydrotomatine and esculeoside A, remains unclear. Comparative SGA profiling was performed in eight tomato accessions, including wild tomato species by HPLC-Fourier transform ion cyclotron resonance mass spectrometry (HPLC-FTICR/MS). On the basis of molecular formulae obtained from accurate m/z and fragmentation patterns by multistage MS/ MS (MS(n)), 123 glycoalkaloids in total were screened. Detailed MS(n) analysis showed that the observed structural diversity was derived from various chemical modifications, such as glycosylation, acetylation, hydroxylation and isomerization. Total SGA content in each tomato accession was in the range of 121-1986 nmol/gfr.wt. Furthermore, the compositional variety of SGA structures was distinctive in some tomato accessions. While most tomato accessions were basically categorized as alpha-tomatine-rich or esculeoside A-rich group, other specific SGAs also accumulated at high levels in wild tomato. Here, five such SGAs were isolated and their structures were determined by NMR spectroscopic analysis, indicating three of them were presumably synthesized during alpha-tomatine metabolism.


