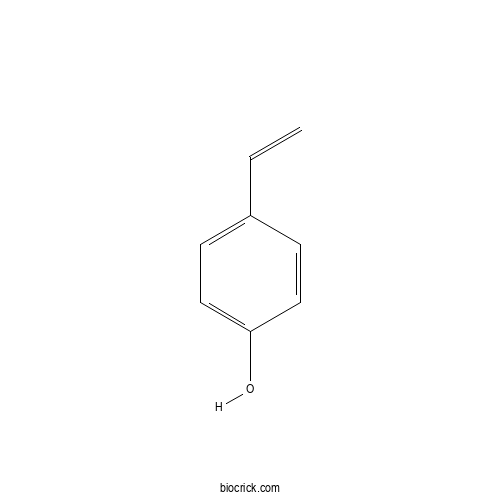
4-VinylphenolCAS# 2628-17-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2628-17-3 | SDF | Download SDF |
| PubChem ID | 62453.0 | Appearance | Powder |
| Formula | C8H8O | M.Wt | 120.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-ethenylphenol | ||
| SMILES | C=CC1=CC=C(C=C1)O | ||
| Standard InChIKey | FUGYGGDSWSUORM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O/c1-2-7-3-5-8(9)6-4-7/h2-6,9H,1H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Vinylphenol Dilution Calculator

4-Vinylphenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.3229 mL | 41.6146 mL | 83.2293 mL | 166.4586 mL | 208.0732 mL |
| 5 mM | 1.6646 mL | 8.3229 mL | 16.6459 mL | 33.2917 mL | 41.6146 mL |
| 10 mM | 0.8323 mL | 4.1615 mL | 8.3229 mL | 16.6459 mL | 20.8073 mL |
| 50 mM | 0.1665 mL | 0.8323 mL | 1.6646 mL | 3.3292 mL | 4.1615 mL |
| 100 mM | 0.0832 mL | 0.4161 mL | 0.8323 mL | 1.6646 mL | 2.0807 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydroactindiolide
Catalog No.:BCX1528
CAS No.:15356-74-8
- Isoescin Ie
Catalog No.:BCX1527
CAS No.:1613506-28-7
- (±)-Dihydrokaempferol
Catalog No.:BCX1526
CAS No.:104486-98-8
- Bitalgenin
Catalog No.:BCX1525
CAS No.:2192-25-8
- Luteolin 7-sulfate
Catalog No.:BCX1524
CAS No.:56857-57-9
- Dahurinol
Catalog No.:BCX1523
CAS No.:38908-87-1
- Acetylseneciphylline N-oxide
Catalog No.:BCX1522
CAS No.:123844-00-8
- Nortanshinone
Catalog No.:BCX1521
CAS No.:97399-70-7
- Prosaikogenin A
Catalog No.:BCX1520
CAS No.:99365-21-6
- Resveratrol-4'-O-(6"-galloyl)-β-D-glucopyranoside
Catalog No.:BCX1519
CAS No.:928340-97-0
- Norfuronol
Catalog No.:BCX1518
CAS No.:19322-27-1
- dehydrotomatine
Catalog No.:BCX1517
CAS No.:157604-98-3
- d-Epigalbacin
Catalog No.:BCX1530
CAS No.:84709-25-1
- (+)-Dehydroabietic acid
Catalog No.:BCX1531
CAS No.:1231-75-0
- 31-Norlanostenol
Catalog No.:BCX1532
CAS No.:16910-39-7
- Mexoticin
Catalog No.:BCX1533
CAS No.:18196-00-4
- Ansamitocin P3
Catalog No.:BCX1534
CAS No.:66584-72-3
- Isobutyryl alkannin
Catalog No.:BCX1535
CAS No.:87562-78-5
- Chrysophanol-1-O-β-gentiobioside
Catalog No.:BCX1536
CAS No.:54944-38-6
- Chrysophanol triglucoside
Catalog No.:BCX1537
CAS No.:120181-07-9
- 11-epi-morgroside V
Catalog No.:BCX1538
CAS No.:2146088-12-0
- β-Chamigrenic acid
Catalog No.:BCX1539
CAS No.:1174388-31-8
- Epigalantamine
Catalog No.:BCX1540
CAS No.:1668-85-5
- N-Desmethyl Galanthamine
Catalog No.:BCX1541
CAS No.:41303-74-6
Fractionation and identification of bioactive compounds from a salt marsh plant Sesuvium potrucastrulam (L.) and its antioxidant activity.[Pubmed:38598319]
Nat Prod Res. 2024 Apr 10:1-4.
Sesuvium portulacastrum (L.) is a fast-growing herbaceous perennial and halophyte belonging to the family Aizoaceae. Bioactive compound identification from halophytes is more helpful for the drug development process. The present investigation was to fractionate and identify the bioactive compounds of leaf extracts from Sesuvium potrucastrulam and evaluate their antioxidant potential. The Soxhlet extraction method was used in this study, and column chromatography was done for the partial purification. The bactericidal activity of the fraction was determined using the agar-well diffusion technique, and the effective fraction was analysed by GC-MS. A hydrogen peroxide-reducing assay was carried out on the antioxidant activity of the elite fraction. Five active fractions were collected from the crude extract. Fraction (F3) exhibited promising antibacterial and antioxidant activity. GS-MS analysis suggested the active compounds of the elite fraction are n-Hexadecanoic acid (29.70%), oleic acid (8.08%), octadecatrienoic acid (8.01%), 2-methoxy-4-Vinylphenol (6.65%), tertracosamethyl-cyclododecasiloxane (6.55%), and lineolic acid (4.68%).
Phenolic phytochemistry, in vitro, in silico, in vivo, and mechanistic anti-inflammatory and antioxidant evaluations of Habenaria digitata.[Pubmed:38487169]
Front Pharmacol. 2024 Feb 29;15:1346526.
Excessive and imbalance of free radicals within the body lead to inflammation. The objective of the current research work was to explore the anti-inflammatory and antioxidant potential of the isolated compounds from Habenaria digitata. In this study, the isolated phenolic compounds were investigated for in vitro and in vivo anti-inflammatory potential along with the antioxidant enzyme. The anti-inflammatory and antioxidant potential of the phenolic compounds was assayed via various enzymes like COX-1/2, 5-LOX and ABTS, DPPH, and H(2)O(2) free radical enzyme inhibitory assay. These compounds were also explored for their in vivo antioxidant activity like examining SOD, CAT, GSH-Px, and MDA levels in the brain, heart, and liver. The anti-inflammatory potential was evaluated using the carrageenan-induced pleurisy model in mice. On the basis of initial screening of isolated compounds, the most potent compound was further evaluated for the anti-inflammatory mechanism. Furthermore, the molecular docking study was also performed for the potent compound. The phenolic compounds were isolated and identified by GC-MS/NMR analysis by comparing its spectra to the library spectra. The isolated phenolic compounds from H. digitata were 5-methylpyrimidine-24,4-diol (1), 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one (2), 2-isopropyl-5-methylphenol (3), 3-methoxy-4-Vinylphenol (4), and 2,6-dimethoxy-4-Vinylphenol (5). In in vitro antioxidant assay, the most potent compound was compound 1 having IC(50) values of 0.98, 0.90, and 5 mug/mL against ABTS, DPPH, and H(2)O(2), respectively. Similarly, against COX1/2 and 5-LOX ,compound 1 was again the potent compound with IC(50) values of 42.76, 10.70, and 7.40 mug/mL. Based on the in vitro results, compound 1 was further evaluated for in vivo antioxidant and anti-inflammatory potential. Findings of the study suggest that H. digitata contains active compounds with potential anti-inflammatory and antioxidant effects. These compounds could be screened as drug candidates for pharmaceutical research, targeting conditions associated with oxidative stress and inflammatory conditions in medicinal chemistry and support their ethnomedicinal use for inflammation and oxidative stress.
Plasma metabolites and risk of seven cancers: a two-sample Mendelian randomization study among European descendants.[Pubmed:38433226]
BMC Med. 2024 Mar 4;22(1):90.
BACKGROUND: While circulating metabolites have been increasingly linked to cancer risk, the causality underlying these associations remains largely uninterrogated. METHODS: We conducted a comprehensive 2-sample Mendelian randomization (MR) study to evaluate the potential causal relationship between 913 plasma metabolites and the risk of seven cancers among European-ancestry individuals. Data on variant-metabolite associations were obtained from a genome-wide association study (GWAS) of plasma metabolites among 14,296 subjects. Data on variant-cancer associations were gathered from large-scale GWAS consortia for breast (N = 266,081), colorectal (N = 185,616), lung (N = 85,716), ovarian (N = 63,347), prostate (N = 140,306), renal cell (N = 31,190), and testicular germ cell (N = 28,135) cancers. MR analyses were performed with the inverse variance-weighted (IVW) method as the primary strategy to identify significant associations at Bonferroni-corrected P < 0.05 for each cancer type separately. Significant associations were subjected to additional scrutiny via weighted median MR, Egger regression, MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO), and reverse MR analyses. Replication analyses were performed using an independent dataset from a plasma metabolite GWAS including 8,129 participants of European ancestry. RESULTS: We identified 94 significant associations, suggesting putative causal associations between 66 distinct plasma metabolites and the risk of seven cancers. Remarkably, 68.2% (45) of these metabolites were each associated with the risk of a specific cancer. Among the 66 metabolites, O-methylcatechol sulfate and 4-Vinylphenol sulfate demonstrated the most pronounced positive and negative associations with cancer risk, respectively. Genetically proxied plasma levels of these two metabolites were significantly associated with the risk of lung cancer and renal cell cancer, with an odds ratio and 95% confidence interval of 2.81 (2.33-3.37) and 0.49 (0.40-0.61), respectively. None of these 94 associations was biased by weak instruments, horizontal pleiotropy, or reverse causation. Further, 64 of these 94 were eligible for replication analyses, and 54 (84.4%) showed P < 0.05 with association patterns consistent with those shown in primary analyses. CONCLUSIONS: Our study unveils plausible causal relationships between 66 plasma metabolites and cancer risk, expanding our understanding of the role of circulating metabolites in cancer genetics and etiology. These findings hold promise for enhancing cancer risk assessment and prevention strategies, meriting further exploration.
A New Phenolic Acid Decarboxylase from the Brown-Rot Fungus Neolentinus lepideus Natively Decarboxylates Biosourced Sinapic Acid into Canolol, a Bioactive Phenolic Compound.[Pubmed:38391667]
Bioengineering (Basel). 2024 Feb 14;11(2):181.
Rapeseed meal (RSM) is a cheap, abundant and renewable feedstock, whose biorefinery is a current challenge for the sustainability of the oilseed sector. RSM is rich in sinapic acid (SA), a p-hydroxycinnamic acid that can be decarboxylated into canolol (2,6-dimethoxy-4-Vinylphenol), a valuable bioactive compound. Microbial phenolic acid decarboxylases (PADs), mainly described for the non-oxidative decarboxylation of ferulic and p-coumaric acids, remain very poorly documented to date, for SA decarboxylation. The species Neolentinus lepideus has previously been shown to biotransform SA into canolol in vivo, but the enzyme responsible for bioconversion of the acid has never been characterized. In this study, we purified and characterized a new PAD from the canolol-overproducing strain N. lepideus BRFM15. Proteomic analysis highlighted a sole PAD-type protein sequence in the intracellular proteome of the strain. The native enzyme (NlePAD) displayed an unusual outstanding activity for decarboxylating SA (V(max) of 600 U.mg(-1), k(cat) of 6.3 s(-1) and k(cat)/K(M) of 1.6 s(-1).mM(-1)). We showed that NlePAD (a homodimer of 2 x 22 kDa) is fully active in a pH range of 5.5-7.5 and a temperature range of 30-55 degrees C, with optima of pH 6-6.5 and 37-45 degrees C, and is highly stable at 4 degrees C and pH 6-8. Relative ratios of specific activities on ferulic, sinapic, p-coumaric and caffeic acids, respectively, were 100:24.9:13.4:3.9. The enzyme demonstrated in vitro effectiveness as a biocatalyst for the synthesis of canolol in aqueous medium from commercial SA, with a molar yield of 92%. Then, we developed processes to biotransform naturally-occurring SA from RSM into canolol by combining the complementary potentialities of an Aspergillus niger feruloyl esterase type-A, which is able to release free SA from the raw meal by hydrolyzing its conjugated forms, and NlePAD, in aqueous medium and mild conditions. NlePAD decarboxylation of biobased SA led to an overall yield of 1.6-3.8 mg canolol per gram of initial meal. Besides being the first characterization of a fungal PAD able to decarboxylate SA, this report shows that NlePAD is very promising as new biotechnological tool to generate biobased vinylphenols of industrial interest (especially canolol) as valuable platform chemicals for health, nutrition, cosmetics and green chemistry.
Characterization of aroma-active compounds in sesame hulls at different roasting temperatures by SAFE and GC-O-MS.[Pubmed:38384683]
Food Chem X. 2024 Feb 9;21:101203.
The study characterized the aroma-active compounds produced by sesame hulls at three roasting temperatures and analyzed the similarities and differences in the aroma profile of sesame hulls with whole seeds and kernels after roasting. Roasting hulls produced mainly furans, aldehydes, and ketones volatiles. 140 Compounds were identified as aroma-active compounds, including 36 key aroma compounds (odor activity value, OAV >/= 1). Among them, furanone (caramel-like, OAV = 80), 3-methylbutanal (fruity, OAV = 124), and 2-methoxy-4-Vinylphenol (burnt, smoky, OAV = 160) gave hulls (180 degrees C) sweet, burnt, and smoky aroma. Due to the contribution of vanillin (fatty, sweet milk, OAV = 45), 2-hydroxy-3-butanone (caramel-like, roast, OAV = 46), and 2-methoxy-4-Vinylphenol (OAV = 78), hulls (200 degrees C) shown strong sweet and roast note. These results identified compounds that contributed significantly to the aroma of sesame hulls and elucidated the contribution of sesame hulls to the flavor of roasted whole seeds and sesame oil.
Study on the Relationship between the Structure and Pyrolysis Characteristics of Lignin Isolated from Eucalyptus, Pine, and Rice Straw through the Use of Deep Eutectic Solvent.[Pubmed:38202802]
Molecules. 2023 Dec 30;29(1):219.
Understanding the pyrolysis product distributions of deep eutectic solvent (DES)-isolated lignins (DESLs) from different types of biomass is of great significance for lignin valorization. The structure and pyrolysis properties of DESLs obtained from eucalyptus (E-DESL), pine (P-DESL), and rice straw (R-DESL) were studied through the use of various methods such as elemental analysis, GPC, HS-GC, and NMR techniques, and the pyrolysis characteristics and product distributions of the DESLs were also further investigated through the use of TGA, Py-GC/MS, and tubular furnace pyrolysis. DESLs with high purity (88.5-92.7%) can be efficiently separated from biomass while cellulose is retained. E-DESL has a relatively low molecular weight, and P-DESL has a relatively higher hydrogen-carbon effective ratio and a lower number of condensation structures. The Py-GC/MS results show that, during DESL pyrolysis, the monomeric aromatic hydrocarbons, p-hydroxyphenyl-type phenols, and catechol-type phenols are gradually released when the guaiacyl-type phenols and syringyl-type phenols decrease with the rising temperature. 4-methylguaiacol and 4-methylcatechol, derived from the guaiacyl-type structural units, are positively correlated with temperature, which causes a significant increase in products with a side-chain carbon number of 1 from P-DESL pyrolysis. 4-Vinylphenol, as a representative product of the R-DESL, derived from p-hydroxyphenyl-type structural units, also gradually increased. In addition, the P-DESL produces more bio-oil during pyrolysis, while gases have the highest distribution in E-DESL pyrolysis. It is of great significance to study the characteristic product distribution of lignin isolated through the use of DES for lignin directional conversion into specific high-value aromatic compounds.
Metabolomic Signatures Differentiate Immune Responses in Avian Influenza Vaccine Recipients.[Pubmed:38181048]
J Infect Dis. 2024 Jan 5:jiad611.
BACKGROUND: Avian influenza viruses pose significant risk to human health. Vaccines targeting the hemagglutinin of these viruses are poorly immunogenic without the use of adjuvants. METHODS: Twenty healthy men and women (18-49 years of age) were randomized to receive two doses of inactivated influenza A/H5N1 vaccine alone (IIV) or with AS03 adjuvant (IIV-AS03) one month apart. Urine and serum samples were collected on day 0 and on days 1, 3, and 7 following first vaccination and subjected to metabolomics analyses to identify metabolites, metabolic pathways, and metabolite clusters associated with immunization. RESULTS: Seventy-three differentially abundant (DA) serum and 88 urine metabolites were identified for any post-vaccination day comparison. Pathway analysis revealed enrichment of tryptophan, tyrosine and nicotinate metabolism in urine and serum among IIV-AS03 recipients. Increased urine abundance of 4-Vinylphenol sulfate on Day 1 was associated with serologic response based on hemagglutination inhibition responses. In addition, 9 DA urine metabolites were identified in participants with malaise compared to those without. CONCLUSIONS: Our findings suggest that tryptophan, tyrosine, and nicotinate metabolism are upregulated among IIV-AS03 recipients compared with IIV alone. Metabolites within these pathways may serve as measures of immunogenicity and may provide mechanistic insights for adjuvanted vaccines.
Creating Remarkably Moisture- and Air-Stable Macromolecular Lewis Acid by Integrating Borane within the Polymer Chain: A Highly Active Catalyst for Homo(co)polymerization of Epoxides.[Pubmed:38155561]
Angew Chem Int Ed Engl. 2024 Feb 12;63(7):e202318645.
Borane-based Lewis acids (LA) play an indispensable role in the Lewis pair (LP) mediated polymerization. However, most borane-based LPs are moisture- and air-sensitive. Therefore, development of moisture and air-stable borane-based LP is highly desirable. To achieve this goal, the concept of "aggregation induced enlargement effects" by chemically linking multiple borane within a nanoscopic confinement was conceived to create macromolecular LA. Accordingly, an extremely moisture and air stable macromolecular borane, namely, PVP-1B featuring poly(4-Vinylphenol) backbone, was constructed. The concentration of borane active site is greatly higher than average concentration due to local confinement. Therefore, an enhanced activity was observed. Moreover, the local LA aggregation effects allow its tolerance to air and large amount of chain transfer agent. Consequently, PVP-1B showed remarkable efficiency for propylene oxide (PO) polymerization at 25 degrees C (TOF=27900 h(-1) ). Furthermore, it enables generation of well-defined telechelic poly (CHO-alt-CO(2) ) diol (0.6-15.3 kg/mol) with narrow Ds via copolymerizing cyclohexene oxide and CO(2) at 80 degrees C. This work indicates unifying multiple borane within a polymer in a macromolecular level shows superior catalytic performance than constructing binary, bi(multi)functional systems in a molecular level. This paves a new way to make functional polyethers.
Selenium content, chemical composition and volatile components of essential oil and hydrosol from flowers of Cardamine violifolia.[Pubmed:38116867]
Chem Biodivers. 2024 Feb;21(2):e202301428.
Cardamine violifolia is a unique selenium hyperaccumulating vegetable in China, but its flowers are commonly wasted in large-scale cultivation. To better utilize this resource, this study explored the selenium content, chemical composition, and volatile organic compounds (VOCs) of hydro-distilling essential oil (EO) and hydrosol from C. violifolia flowers. ICP-MS results indicated that the EO and hydrosol contained selenium reaching 13.66+/-2.82 mg/kg and 0.0084+/-0.0013 mg/kg, respectively. GC-MS analysis revealed that organic acids, hydrocarbons, and amines were the main components of EO. Additionally, benzyl nitrile, benzaldehyde, benzyl isothiocyanate, benzyl alcohol, megastigmatrienone, and 2-methoxy-4-Vinylphenol also existed in considerable amounts. The hydrosol extract had fewer components, mainly amines. HS-SPME-GC-MS corresponded to the composition analysis and aromatic compounds were the prevalent VOCs, while HS-GC-IMS primarily identified C2-C10 molecular alcohols, aldehydes, ethers, and sulfur-containing compounds. This study first described the chemical composition and VOC profiles of EO and hydrosol from selenium hyperaccumulating plant.
Anti-Ulcerative Colitis Effects and Active Ingredients in Ethyl Acetate Extract from Decoction of Sargentodoxa cuneata.[Pubmed:38005385]
Molecules. 2023 Nov 19;28(22):7663.
Ulcerative colitis (UC) is an intractable disease prevalent worldwide. While ethyl acetate extract from decoction of Sargentodoxa cuneata (EAdSc) has potential anti-inflammatory activity, its effects on UC remain unknown. In this study, the constituent compounds discussed in the literature and identified by gas chromatography and mass spectrometry (GC-MS) were collected, and the blood-soluble components of EAdSc were identified by liquid chromatography-mass spectrometry. The network pharmacology analysis and molecular docking analysis were performed to explore the potential underlying mechanism and active ingredients of EAdSc against UC. Furthermore, mice with dextran sulfate sodium (DSS)-induced UC were used to study the therapeutic effects and validate the mechanism of EAdSc against UC. A total of 53 compounds from EAdSc were identified in the literature and by GC-MS, and 22 blood-soluble EAdSc components were recognized. Network pharmacology analysis revealed that multiple inflammatory signaling pathways are involved in EAdSc's anti-UC activity. Furthermore, molecular docking analysis showed that the eleutheroside A, liriodendrin, epicatechin, 2-methoxy-4-Vinylphenol, catechin, androsin, coumaroyltyramine, and catechol may be active against UC through the TLR4/NF-kappaB/NLRP3 pathway. EAdSc reduced the disease activity, macroscopic colon damage, and histological damage indices, as well as inhibiting DSS-induced spleen enlargement and colon shortening. In addition, EAdSc decreased the levels of tumor necrosis factor-alpha (TNF-alpha), interleukin (IL)-1beta, IL-6, and IL-17, as well as the expression of TLR4, NF-kappaB p65, NLRP3, and Caspase-1 mRNA in colon tissues. These results provide insights into the anti-UC effects and underlying mechanisms of EAdSc and help elucidate the active ingredients of EAdSc in the treatment of UC.
Challenges and advances in biotechnological approaches for the synthesis of canolol and other vinylphenols from biobased p-hydroxycinnamic acids: a review.[Pubmed:37964324]
Biotechnol Biofuels Bioprod. 2023 Nov 14;16(1):173.
p-Hydroxycinnamic acids, such as sinapic, ferulic, p-coumaric and caffeic acids, are among the most abundant phenolic compounds found in plant biomass and agro-industrial by-products (e.g. cereal brans, sugar-beet and coffee pulps, oilseed meals). These p-hydroxycinnamic acids, and their resulting decarboxylation products named vinylphenols (canolol, 4-vinylguaiacol, 4-Vinylphenol, 4-vinylcatechol), are bioactive molecules with many properties including antioxidant, anti-inflammatory and antimicrobial activities, and potential applications in food, cosmetic or pharmaceutical industries. They were also shown to be suitable precursors of new sustainable polymers and biobased substitutes for fine chemicals such as bisphenol A diglycidyl ethers. Non-oxidative microbial decarboxylation of p-hydroxycinnamic acids into vinylphenols involves cofactor-free and metal-independent phenolic acid decarboxylases (EC 4.1.1 carboxyl lyase family). Historically purified from bacteria (Bacillus, Lactobacillus, Pseudomonas, Enterobacter genera) and some yeasts (e.g. Brettanomyces or Candida), these enzymes were described for the decarboxylation of ferulic and p-coumaric acids into 4-vinylguaiacol and 4-Vinylphenol, respectively. The catalytic mechanism comprised a first step involving p-hydroxycinnamic acid conversion into a semi-quinone that then decarboxylated spontaneously into the corresponding vinyl compound, in a second step. Bioconversion processes for synthesizing 4-vinylguaiacol and 4-Vinylphenol by microbial decarboxylation of ferulic and p-coumaric acids historically attracted the most research using bacterial recombinant phenolic acid decarboxylases (especially Bacillus enzymes) and the processes developed to date included mono- or biphasic systems, and the use of free- or immobilized cells. More recently, filamentous fungi of the Neolentinus lepideus species were shown to natively produce a more versatile phenolic acid decarboxylase with high activity on sinapic acid in addition to the others p-hydroxycinnamic acids, opening the way to the production of canolol by biotechnological processes applied to rapeseed meal. Few studies have described the further microbial/enzymatic bioconversion of these vinylphenols into valuable compounds: (i) synthesis of flavours such as vanillin, 4-ethylguaiacol and 4-ethylphenol from 4-vinylguaiacol and 4-Vinylphenol, (ii) laccase-mediated polymer synthesis from canolol, 4-vinylguaiacol and 4-Vinylphenol.
Interaction between a Commercial Mannoprotein and Cyanidin-3-O-glucoside-4-vinylphenol and Its Stability and Antioxidative Properties as a Novel Functional Pigment.[Pubmed:37910136]
J Agric Food Chem. 2023 Nov 1.
Hydroxyphenyl-pyranoanthocyanins, which are derived from anthocyanins and phenolic acids during the fermentation and aging of red wine, are prone to polymerization and precipitation, which largely limits their application and bioactivity research. In the present study, cyanidin-3-O-glucoside-4-Vinylphenol (C3GVP), a hydroxyphenyl-pyranoanthocaynin, was prepared from C3G and p-coumaric acid, and mannoprotein (MP) was employed to improve its stability in various complex solvents by forming a stable anthocyanin-MP complex. We used scanning electron microscopy, ultraviolet-visible spectroscopy, Fourier-transform infrared spectroscopy, and circular dichroism spectroscopy to observe structural changes in C3GVP and MP. The results demonstrated that the intermolecular polymerization of C3GVP was mitigated and the secondary conformation of MP was changed slightly. Fluorescence spectroscopy and molecular docking indicated that C3GVP and MP interacted via hydrogen bonds and hydrophobic interactions. Importantly, the C3GVP-MP complex exhibited better thermal stability and antioxidant capacity than C3G.
Dielectric constant enhancement of poly 4-vinylphenol (PVPh) via graphene flakes incorporation through electrospray atomization for energy storage.[Pubmed:37901272]
RSC Adv. 2023 Oct 27;13(45):31426-31434.
We report on the fabrication of hybrid composite poly 4-vinlyphenol (PVPh)/graphene thin film via cost-effective electrospray atomization deposition technique. Thin films fabricated through manipulating deposition technique in two different ways which are blending and layer by layer (LBL). For investigation of PVPh/graphene hybrid composite dielectric behavior in comparison to PVPh; three asymmetric MIS thin film capacitors were fabricated, where dielectric thin films (i) PVPh and (ii & iii) hybrid composite thin films PVPh/graphene (blended and LBL) were sandwiched between electrodes i.e. indium tin oxide (ITO) and p-type semiconductor poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS). The dielectric properties of the thin films were characterized for frequencies 1 to 100 kHz while utilizing the MIS thin film capacitors. The capacitance obtained at 1 kHz frequency for PVPh/graphene (LBL) dielectric layer at the voltage range +/-10 volts was 8.5 mF cm(-2) while for blended PVPh/graphene thin film the capacitance at the voltage range +/-3 volts was 0.40 muF cm(-2) and for pristine PVPh as dielectric layer the capacitance at voltage range +/-1 volts was 1.45 muF cm(-2). Similarly, even at higher frequencies up to 100 kHz, the PVPh/graphene (LBL) showed stable behavior. Thus, the composite PVPh/graphene (LBL) thin film has a better dielectric nature compared to the composite PVPh/graphene (blended) thin film, even at higher frequencies with larger operational voltage window. This distinguishing nature of the composite PVP/graphene (LBL) is attributed to increase in dielectric constant due to graphene flakes in between PVPh. For the thin films LBL and blended PVPh/graphene, the calculated dielectric constant at 10 kHz is 6.7 and 0.023 while at 100 kHz it is 2 and 0.0167, respectively.
Phytochemical characterization, anti-diarrhoeal, analgesic, anti-inflammatory activities and toxicity profile of Ananas comosus (L.) Merr (pineapple) leaf in albino rats.[Pubmed:37748634]
J Ethnopharmacol. 2024 Jan 30;319(Pt 2):117224.
ETHNOPHARMACOLOGICAL RELEVANCE: Ananas comosus (pineapple) leaf is used in ethnomedicine to treat diarrhoea, inflammation, pain, bacterial infections and oedema. AIM OF THE STUDY: The aim of this study was to investigate the anti-diarrhoeal, anti-inflammatory and analgesic effects as well as the toxicity profile of the aqueous Ananas comosus leaf extract (AACLE) in rats. METHODS: Methanol ACLE was subjected to gas chromatography-mass spectrometry (GC-MS) analysis. In the acute toxicity study, a single oral dose of up to 5000 mg/kg AACLE was administered. In the subacute toxicity study (28 days), rats in groups 2-4 received AACLE orally. The anti-diarrhoeal effect was studied using charcoal meal and castor oil-induced diarrhoea. Anti-inflammatory and analgesic tests were measured using egg albumin-induced paw oedema and acetic acid-induced writhing methods, respectively. For the subacute toxicity, anti-diarrhoeal, analgesic, and anti-inflammatory studies, AACLE was administered orally to rats at doses of 200, 400, and 600 mg/kg body weight. RESULTS: Hexadecanoic acid methyl ester, 2-methoxy-4-Vinylphenol, n-hexadecanoic acid and n-heptadecanol-1 were identified among other compounds with known pharmacological activities by GC-MS analysis. No deaths, behavioural changes, or signs of toxicity were observed in the acute toxicity study. Treatment with AACLE (28 days) decreased aspartate aminotransferase, alanine transaminase, total cholesterol, triglycerides, and low-density lipoprotein cholesterol, while high-density lipoprotein cholesterol, glutathione, and catalase increased when compared with control (P < 0.05). Treatment with AACLE did not cause significant changes in haematological or renal function parameters. Treatment with AACLE inhibited gastrointestinal motility and castor oil-induced diarrhoea in rats. Treatment with AACLE resulted in a dose-dependent (200-600 mg/kg) significant (P < 0.05) anti-diarrhoeal, analgesic, and anti-inflammatory effect compared to standard drugs. CONCLUSION: Our study revealed that ACLE is not toxic but contains bioactive compounds with anti-diarrhoeal, anti-inflammatory, antimicrobial, and hepatoprotective activity. AACLE has antidiarrhoeal, analgesic and anti-inflammatory activity in rats, which justifies its therapeutic use in traditional medicine.
Aroma characteristics of flaxseed milk via GC-MS-O and odor activity value calculation: Imparts and selection of different flaxseed varieties.[Pubmed:37657337]
Food Chem. 2024 Jan 30;432:137095.
Currently, the effect of varieties on the flavor and stability of flaxseed milk remains unknown. Therefore, this study was conducted to investigate the effects of different varieties on the stability, sensory, and aromas of flaxseed milk. 51 volatile compounds were identified in flaxseed milk using Stir Bar Sorptive Extraction-Gas Chromatography-Olfactometry-Mass Spectrometry (SBSE-GC-O-MS). Among them, 1-octen-3-ol, 2-methoxy-4-Vinylphenol, and 2,3,5-trimethylpyrazine contributed higher relative odor active values (ROAV), resulted in the fruity, roasted, sweet, and cucumber in flaxseed milk. Isovaleraldehyde (green notes) was not detected in XHZM. However, other compounds such as 1-nonanol (floral), gamma-nonanolactone and gamma-octanoic lactone (coconut milk) had higher concentrations, causing a better sensory evaluation. Additionally, its stability was relatively good. The orthogonal partial least-squares regression (OPLS) model and VIP values showed that eight compounds were responsible for the sensory differences from different varietals. The study provided references to selection and understanding flavor composition basis of flaxseed milk.


