EpigalantamineCAS# 1668-85-5 |
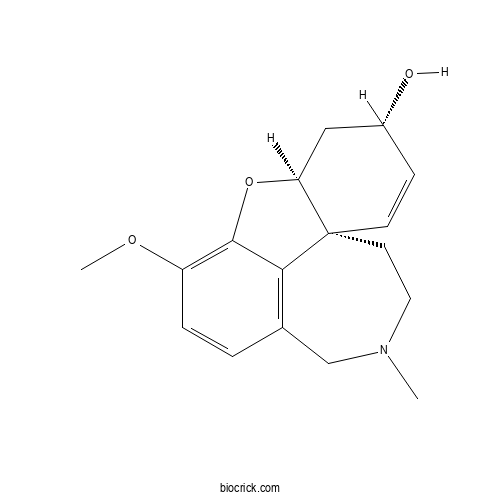
- Galantamine
Catalog No.:BCN2868
CAS No.:357-70-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1668-85-5 | SDF | Download SDF |
| PubChem ID | 676392.0 | Appearance | Powder |
| Formula | C17H21NO3 | M.Wt | 287.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,12S,14S)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-14-ol | ||
| SMILES | CN1CCC23C=CC(CC2OC4=C(C=CC(=C34)C1)OC)O | ||
| Standard InChIKey | ASUTZQLVASHGKV-IFIJOSMWSA-N | ||
| Standard InChI | InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14+,17+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Epigalantamine Dilution Calculator

Epigalantamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.48 mL | 17.3998 mL | 34.7996 mL | 69.5991 mL | 86.9989 mL |
| 5 mM | 0.696 mL | 3.48 mL | 6.9599 mL | 13.9198 mL | 17.3998 mL |
| 10 mM | 0.348 mL | 1.74 mL | 3.48 mL | 6.9599 mL | 8.6999 mL |
| 50 mM | 0.0696 mL | 0.348 mL | 0.696 mL | 1.392 mL | 1.74 mL |
| 100 mM | 0.0348 mL | 0.174 mL | 0.348 mL | 0.696 mL | 0.87 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- β-Chamigrenic acid
Catalog No.:BCX1539
CAS No.:1174388-31-8
- 11-epi-morgroside V
Catalog No.:BCX1538
CAS No.:2146088-12-0
- Chrysophanol triglucoside
Catalog No.:BCX1537
CAS No.:120181-07-9
- Chrysophanol-1-O-β-gentiobioside
Catalog No.:BCX1536
CAS No.:54944-38-6
- Isobutyryl alkannin
Catalog No.:BCX1535
CAS No.:87562-78-5
- Ansamitocin P3
Catalog No.:BCX1534
CAS No.:66584-72-3
- Mexoticin
Catalog No.:BCX1533
CAS No.:18196-00-4
- 31-Norlanostenol
Catalog No.:BCX1532
CAS No.:16910-39-7
- (+)-Dehydroabietic acid
Catalog No.:BCX1531
CAS No.:1231-75-0
- d-Epigalbacin
Catalog No.:BCX1530
CAS No.:84709-25-1
- 4-Vinylphenol
Catalog No.:BCX1529
CAS No.:2628-17-3
- Dihydroactindiolide
Catalog No.:BCX1528
CAS No.:15356-74-8
- N-Desmethyl Galanthamine
Catalog No.:BCX1541
CAS No.:41303-74-6
- Anemarrhenasaponin Ia
Catalog No.:BCX1542
CAS No.:221317-02-8
- Momordin IIc
Catalog No.:BCX1543
CAS No.:96990-19-1
- Oleanolic acid -3-O-glucosyl(1-2)xylyl(1-3)glucosiduronic acid
Catalog No.:BCX1544
CAS No.:1447508-78-2
- 7α-Hydroxycholesterol
Catalog No.:BCX1545
CAS No.:566-26-7
- 8-Methyl Chrysophanol
Catalog No.:BCX1546
CAS No.:3300-25-2
- Ursonic acid methyl ester
Catalog No.:BCX1547
CAS No.:989-72-0
- Rotundifuran
Catalog No.:BCX1548
CAS No.:50656-65-0
- Lactiflorin
Catalog No.:BCX1549
CAS No.:1361049-59-3
- Dihydrolapachenole
Catalog No.:BCX1550
CAS No.:20213-26-7
- Sibiricaxanthone A
Catalog No.:BCX1551
CAS No.:241125-76-8
- Ebeiedinone
Catalog No.:BCX1552
CAS No.:25650-68-4
High-performance liquid chromatographic method with UV photodiode-array, fluorescence and mass spectrometric detection for simultaneous determination of galantamine and its phase I metabolites in biological samples.[Pubmed:17416214]
J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Jun 15;853(1-2):265-74.
Galantamine, an alkaloid isolated from the bulbs and flowers of Caucasian snowdrop (Galanthus woronowii, Amaryllidaceae) and related species, is employed in human medicine for the treatment of various neuromuscular and neurodegenerative diseases. After the administration, the products of oxidative biotransformation (O-desmethyl-galantamine, N-desmethyl-galantamine, galantamine-N-oxide) and chiral conversion (Epigalantamine) are formed in various concentrations from parent compound. For the identification and determination of galantamine and its phase I metabolites in blood plasma and tissues, a new bioanalytical method based on a reversed-phase high-performance liquid chromatography with UV photodiode-array, fluorescence and mass spectrometric detection was developed, validated and applied to pharmacokinetic and biotransformation studies. Sample preparation included a homogenization of the rat tissues (liver, brain, hypophysis) in a phosphate buffer 0.05 mol/L pH 7.4. Plasma samples and tissue homogenates were purified using a mixed-mode solid-phase extraction (Waters Oasis MCX cartridges). Galantamine, its above-mentioned metabolites and the internal standard codeine were separated on a Discovery HS F5 column (Supelco, 150 mmx4.6 mm I.D., 5 microm) at flow rate of 1 mL/min using a linear gradient elution. UV photodiode-array and mass spectrometric detection were employed for the identification of individual galantamine metabolites in various biomatrices, the fluorescence detection (lambdaexcit=280 nm/lambdaemiss=310 nm) was chosen for the quantification of galantamine and its metabolites. The developed method was applicable in liver tissue in the range from 0.50 to 63.47 nmol/g of galantamine, from 0.32 to 41.42 nmol/g of O-desmethyl-galantamine, from 0.54 to 69.40 nmol/g of N-desmethyl-galantamine and from 0.70 to 89.03 nmol/g of Epigalantamine. Limit of detection was found to be 0.04 nmol/g for galantamine, 0.19 nmol/g for O-desmethyl-galantamine, and 0.07 nmol/g for N-desmethyl-galantamine and Epigalantamine.
In vitro metabolism of galanthamine hydrobromide (Nivalin) by rat and rabbit liver homogenate.[Pubmed:3609070]
Eur J Drug Metab Pharmacokinet. 1987 Jan-Mar;12(1):25-30.
The metabolism of galanthamine hydrobromide (Nivalin) was investigated in rat and rabbit liver homogenates. Experiments were carried out varying several parameters of incubation: substrate (galanthamine hydrobromide, galanthamine, galanthaminone and epigalanthamine), cofactor enrichment (NADPH, NADP/G-6-P, NAD), pH (7.4 and 9.3), time of incubation. Substrates and metabolites were identified and quantitatively determined by GC/MS. In vitro metabolism in rat liver homogenate was negligible. The experiments with rabbit liver homogenate indicated, that galanthamine was actively metabolised the major metabolites being the oxidised product - galanthaminone, and the isomer of galanthamine - Epigalantamine. The experimental results show that the metabolism of galanthamine is substrate and product stereoselective.


