2',4',6'-TrimethoxyacetophenoneCAS# 832-58-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 832-58-6 | SDF | Download SDF |
| PubChem ID | 123089 | Appearance | Powder |
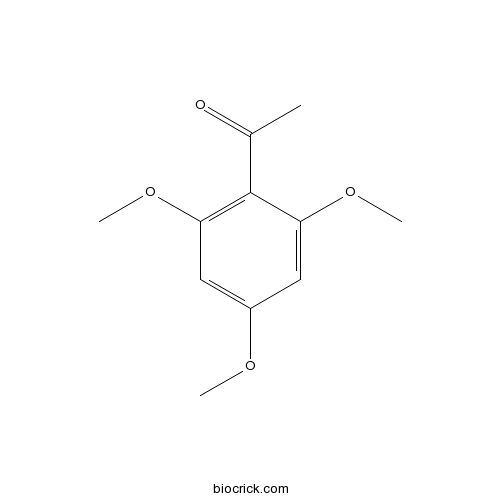
| Formula | C11H14O4 | M.Wt | 210.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(2,4,6-trimethoxyphenyl)ethanone | ||
| SMILES | CC(=O)C1=C(C=C(C=C1OC)OC)OC | ||
| Standard InChIKey | KPZWHZSIXZXDMW-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Phytochemistry (Oxford), 1989, 28(11):3193-3196.Free and glucosyloxy acetophenones from Pancratium biflorum.[Reference: WebLink]
|

2',4',6'-Trimethoxyacetophenone Dilution Calculator

2',4',6'-Trimethoxyacetophenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7574 mL | 23.7869 mL | 47.5737 mL | 95.1475 mL | 118.9343 mL |
| 5 mM | 0.9515 mL | 4.7574 mL | 9.5147 mL | 19.0295 mL | 23.7869 mL |
| 10 mM | 0.4757 mL | 2.3787 mL | 4.7574 mL | 9.5147 mL | 11.8934 mL |
| 50 mM | 0.0951 mL | 0.4757 mL | 0.9515 mL | 1.9029 mL | 2.3787 mL |
| 100 mM | 0.0476 mL | 0.2379 mL | 0.4757 mL | 0.9515 mL | 1.1893 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Angelol G
Catalog No.:BCN7963
CAS No.:83199-38-6
- 8alpha-Tigloyloxyhirsutinolide 13-O-acetate
Catalog No.:BCN7108
CAS No.:83182-58-5
- Kaempferol 3-O-rhamninoside
Catalog No.:BCN6845
CAS No.:83170-31-4
- Bonducellin
Catalog No.:BCN6823
CAS No.:83162-84-9
- Angelol B
Catalog No.:BCN8037
CAS No.:83156-04-1
- Carpalasionin
Catalog No.:BCN7644
CAS No.:83150-97-4
- Octreotide acetate
Catalog No.:BCC5643
CAS No.:83150-76-9
- (+)-Sophoridine
Catalog No.:BCC8360
CAS No.:83148-91-8
- 3-Hydroxymethylenetanshinquinone
Catalog No.:BCN2492
CAS No.:83145-47-5
- 4',5-Dihydroxy-3',5',6,7-tetramethoxyflavone
Catalog No.:BCN1335
CAS No.:83133-17-9
- 5-Hydroxymethyl-7-methoxybenzofuran
Catalog No.:BCN4372
CAS No.:831222-78-7
- (-)-Epigallocatechin-3-(3''-O-methyl) gallate
Catalog No.:BCN1336
CAS No.:83104-87-4
- Astragaloside A
Catalog No.:BCC6494
CAS No.:83207-58-3
- ISX 9
Catalog No.:BCC6181
CAS No.:832115-62-5
- 1beta-Hydroxy-beta-eudesmol
Catalog No.:BCN7097
CAS No.:83217-89-4
- Dehydrocavidine
Catalog No.:BCN2549
CAS No.:83218-34-2
- Angelol H
Catalog No.:BCN8047
CAS No.:83247-73-8
- APD668
Catalog No.:BCC5389
CAS No.:832714-46-2
- LF 11
Catalog No.:BCC6174
CAS No.:832729-13-2
- Lauryl-LF 11
Catalog No.:BCC6175
CAS No.:832729-14-3
- Napabucasin
Catalog No.:BCC6525
CAS No.:83280-65-3
- Menisporphine
Catalog No.:BCN7902
CAS No.:83287-02-9
- 7-Hydroxycoumarin-6-carboxylic acid
Catalog No.:BCN4375
CAS No.:833-52-3
- Bryostatin 1
Catalog No.:BCC7343
CAS No.:83314-01-6
Bioaccumulation, maternal transfer and effects of dietary 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) exposure on medaka fish (Oryzias latipes) offspring.[Pubmed:28987991]
Aquat Toxicol. 2017 Nov;192:241-250.
A previous study conducted in our laboratory with growing medaka (Oryzias latipes) showed the capacity of BDE-47 (10-1000ng/g) to bioaccumulate during a 40-day oral exposure. However, the results did not provide evidence for effects during or after the exposure period. In this study, breeding medakas were fed a diet for 40days that contained 1000ng of BDE-47/g. At predefined time points, females (time points 10, 20, 30 and 40), males (time points 30 and 40) and pools of laid eggs (time points 10, 20, 30 and 40) were sampled and collected for: 1) the BDE-47 quantitative analysis in adults in the <24-h-old post-fertilization (hpf) embryos, and in the <24-h-old post-hatch (hph) eleutheroembryos; 2) the evaluation of fecundity, fertility and hatching. Additional pools of embryos collected at time point 40 were evaluated for: 1) the active swimming behavior of the 48hph offspring in the eleutheroembryonic stage; 2) the BDE-47 quantification in the 240hph resultant larvae. BDE-47 accumulated in parents rapidly, and concentrations remained constant at higher levels in males (values within the 50-60ng/g wet weight -w.w.- range) compared with females (70ng/g w.w. range). The BDE-47 concentrations detected in embryos and eleutheroembryos ranged from 200 to 500ng/g w.w. for time points 10-40. Reproductive capacity, hatching and ensuing swim bladder inflation were not affected by parental BDE-47 dietary exposure, nor was the active swimming behavior in eleutheroembryos. The BDE-47 concentration in the 240hph larvae lowered to levels close to those detected in parents. Despite the efficient BDE-47 maternal transfer, these results offered no evidence for BDE-47 effects on fish reproduction or in the early life stages of offspring.
Orange Fluorescent Ru(III) Complexes Based on 4'-Aryl Substituted 2,2':6',2''-Terpyridine for OLEDs Application.[Pubmed:28956219]
J Fluoresc. 2018 Jan;28(1):173-182.
A series of ruthenium (III) complexes of the formulae [Ru(4-Mephtpy)2]Cl3(1) [Ru(L 1 )], [Ru(3,4,5-tmphtpy)2]Cl3(2) [Ru(L 2 )], and [Ru(4-thptpy)2]Cl3(3) [Ru(L 3 )], (where L = terpy = 2.2':6'2'' terpyridine ligands) are synthesized. The complexes were characterized by elemental analyses, spectroscopic and electrochemical data. The density functional theory (DFT) outlines the geometric optimisation and electronic charge transition of these complexes. Photophysical studies describe that the luminescence of Ru(III) complexes is due to electronic transition between the energy levels of singly unoccupied molecular orbitals (SUMO) and singly occupied molecular orbitals (SOMO). It also exhibits the potential charge transfer to pi-pi* and n-pi* states due to MLCT and ILCT processes of the complexes. The observed bands centered at 591 and 620 nm demonstrate that these emissions originated from the transition of SUMO to SOMO energy levels, that is, from the radiative decay from the doublet exciton. Due to the heavy metal effect of Ru(III) ions the photophysical behaviour depends on the MLCT process. In conclusion, that the all three Ru(L 1 -L 3 ) complexes are fallen orange emission.
Copper-Catalyzed Cascade Cyclization of 1,7-Enynes toward Trifluoromethyl-Substituted 1'H-Spiro[azirine-2,4'-quinolin]-2'(3'H)-ones.[Pubmed:28933859]
Org Lett. 2017 Oct 6;19(19):5186-5189.
A novel method for the synthesis of trifluoromethyl-containing 1'H-spiro[azirine-2,4'-quinolin]-2'(3'H)-ones by a CF3-radical-triggered tandem reaction of benzene-linked 1,7-enynes is described. This protocol utilizes 1-trifluoromethyl-1,2-benziodoxole as the trifluoromethylating reagent and TMSN3 as the aminating reagent. By this method, various potentially bioactive trifluoromethylated 1'H-spiro[azirine-2,4'-quinolin]-2'(3'H)-ones were facilely synthesized via a radical cascade process.
4'-C-Methoxy-2'-deoxy-2'-fluoro Modified Ribonucleotides Improve Metabolic Stability and Elicit Efficient RNAi-Mediated Gene Silencing.[Pubmed:28937776]
J Am Chem Soc. 2017 Oct 18;139(41):14542-14555.
We designed novel 4'-modified 2'-deoxy-2'-fluorouridine (2'-F U) analogues with the aim to improve nuclease resistance and potency of therapeutic siRNAs by introducing 4'-C-methoxy (4'-OMe) as the alpha (C4'alpha) or beta (C4'beta) epimers. The C4'alpha epimer was synthesized by a stereoselective route in six steps; however, both alpha and beta epimers could be obtained by a nonstereoselective approach starting from 2'-F U. (1)H NMR analysis and computational investigation of the alpha-epimer revealed that the 4'-OMe imparts a conformational bias toward the North-East sugar pucker, due to intramolecular hydrogen bonding and hyperconjugation effects. The alpha-epimer generally conceded similar thermal stability as unmodified nucleotides, whereas the beta-epimer led to significant destabilization. Both 4'-OMe epimers conferred increased nuclease resistance, which can be explained by the close proximity between 4'-OMe substituent and the vicinal 5'- and 3'-phosphate group, as seen in the X-ray crystal structure of modified RNA. siRNAs containing several C4'alpha-epimer monomers in the sense or antisense strands triggered RNAi-mediated gene silencing with efficiencies comparable to that of 2'-F U.


