2-Chlorocinnamic acidCAS# 3752-25-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

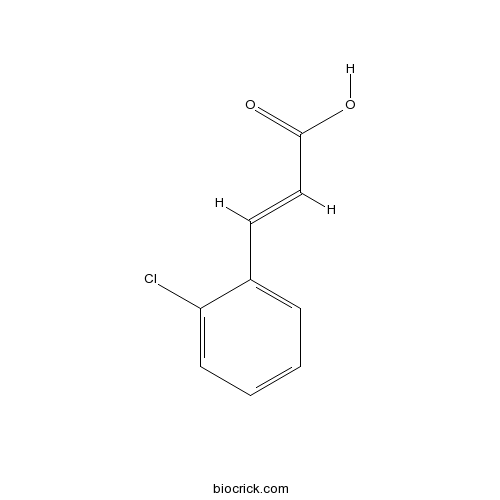
| Cas No. | 3752-25-8 | SDF | Download SDF |
| PubChem ID | 700642 | Appearance | White powder |
| Formula | C9H7ClO2 | M.Wt | 182.61 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(2-chlorophenyl)prop-2-enoic acid | ||
| SMILES | C1=CC=C(C(=C1)C=CC(=O)O)Cl | ||
| Standard InChIKey | KJRRTHHNKJBVBO-AATRIKPKSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2-Chlorocinnamic acid is a photosensitive compound, it can inhibit tyrosinase activity. 2-Chlorocinnamic acid and 4-chlorocinnamic acid show potent urease inhibitory activities with the respective IC50 values of 0.66 and 1.10 uM. |
| In vitro | Photosensitive semiconductor nanocrystals, photosensitive composition comprising semiconductor nanocrystals and method for forming semiconductor nanocrystal pattern using the same[Reference: WebLink]US 8758864 B2[P]. 2014.4. The organic-inorganic hybrid electroluminescent device according to claim 1, wherein the compound containing a photosensitive functional group is selected from a group consisting of methacrylic acid, crotonic acid, vinylacetic acid, tiglic acid, 3,3-dimethylacrylic acid, trans-2-pentenoic acid, 4-pentenoic acid, trans-2-methyl-2-pentenoic acid, 2,2-dimethyl-4-pentenoic acid, trans-2-hexenoic acid, trans-3-hexenoic acid, 2-ethyl-2-hexenoic acid, 6-heptenoic acid, 2-octenoic acid, citronellic acid, undecylenic acid, myristoleic acid, palmitoleic acid, oleic acid, elaidic acid, cis-11-elcosenoic acid, euric acid, nervonic acid, trans-2,4-pentadienoic acid, 2,4-hexadienoic acid, 2,6-heptadienoic acid, geranic acid, linoleic acid, 11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, arachidonic acid, cis-5,8,11,14,17-eicosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, fumaric acid, maleic acid, itaconic acid, ciraconic acid, mesaconic acid, trans-glutaconic acid, trans-beta-hydromuconic acid, trans-traumatic acid, trans-muconic acid, cis-aconitic acid, trans-aconitic acid, cis-3-chloroacrylic acid, trans-3-chloroacrylic acid, 2-bromoacrylic acid, 2-(trifluoromethyl)acryl-ic acid, trans-styrylacetic acid, trans-cinnamic acid, alpha.-methylcinnamic acid, 2-methylcinnamic acid, 2-fluorocinnamic acid, 2-(trifluoromethyl)cinnamic acid, 2-Chlorocinnamic acid, 2-methoxycinnamic acid, 2-hydroxycinnamic acid, 2-nitrocinnamic acid, 2-carboxycinnamic acid, trans-3-fluorocinnamic acid, 3-(trifluoromethyl)cinnamic acid, 3-chlorocinnamic acid, 3-bromocinnamic acid, 3-methoxycinnamic acid, 3-hydroxycinnamic acid, 3-nitrocinnamic acid, 4-methylcinnamic acid, 4-fluorocinnamic acid, trans-4-(Trifluoromethyl)cinnamic acid, 4-chlorocinnamic acid, 4-bromocinnamic acid, 4-methoxycinnamic acid, 4-hydroxycinnamic acid, 4-nitrocinnamic acid, 3,3-dimethoxycinnamic acid, 4-vinylbenzoic acid, allyl methyl sulfide, allyl disulfide, diallyl amine, oleylamine, 3-amino-1-propanol vinyl ether, 4-chlorocinnamonitrile, 4-methoxycinnamonitrile, 3,4-dimethoxycinnamonitrile, 4-dimethylaminocinnamonitrile, acrylonitrile, allyl cyanide, crotononitrile, methacrylonitrile, cis-2-pentenenitrile, trans-3-pentenenitrile, 3,7-dimethyl-2,6-octadienenitrile, and 1,4-dicyano-2-butene. |
| Kinase Assay | Synthesis, Characterization and Biological Evaluation of Two Silver(I) trans‐Cinnamate Complexes as Urease Inhibitors.[Reference: WebLink]Inhibitory kinetics of chlorocinnamic acids on mushroom tyrosinase.[Pubmed: 23958639]J Biosci Bioeng. 2014 Feb;117(2):142-6.Tyrosinase (EC 1.14.18.1) is the key enzyme of most food enzymatic oxidation. Tyrosinase inhibitors are important in food industry. Zeitschrift Für Anorganische Und Allgemeine Chemie,2014, 640(2):423-8.Two new silver(I) trans‐cinnamates, namely [Ag(2‐cca)(H2O)]2 (1) and [Ag(4‐cca)] n (2) (2‐ccaH = 2-Chlorocinnamic acid and 4‐ccaH = 4‐chlorocinnamic acid), were synthesized and structurally characterized. |
| Structure Identification | Chemosphere. 2000 Jun;40(12):1417-25.A novel ortho-dehalogenation reaction of 2-chlorocinnamic acid catalyzed by the pink yeast Rhodotorula rubra Y-1529.[Pubmed: 10789983]In the present study, a resting cells suspension of Rhodotorula rubra Y-1529 was shown to have the capacity to perform an ortho-dehalogenation reaction on 2-Chlorocinnamic acid. |

2-Chlorocinnamic acid Dilution Calculator

2-Chlorocinnamic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4762 mL | 27.3808 mL | 54.7615 mL | 109.523 mL | 136.9038 mL |
| 5 mM | 1.0952 mL | 5.4762 mL | 10.9523 mL | 21.9046 mL | 27.3808 mL |
| 10 mM | 0.5476 mL | 2.7381 mL | 5.4762 mL | 10.9523 mL | 13.6904 mL |
| 50 mM | 0.1095 mL | 0.5476 mL | 1.0952 mL | 2.1905 mL | 2.7381 mL |
| 100 mM | 0.0548 mL | 0.2738 mL | 0.5476 mL | 1.0952 mL | 1.369 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Amikacin
Catalog No.:BCC5206
CAS No.:37517-28-5
- DHP Linker
Catalog No.:BCC2830
CAS No.:3749-36-8
- Metastin (human)
Catalog No.:BCC5761
CAS No.:374683-24-6
- Kisspeptin 10 (human)
Catalog No.:BCC7415
CAS No.:374675-21-5
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- Boc-DL-Ala-OH
Catalog No.:BCC3050
CAS No.:3744-87-4
- DS2
Catalog No.:BCC7748
CAS No.:374084-31-8
- Cephaeline Hydrochloride
Catalog No.:BCC8307
CAS No.:3738-70-3
- Decloxizine
Catalog No.:BCC5529
CAS No.:3733-63-9
- 3-Quinuclidinone
Catalog No.:BCC8642
CAS No.:3731-38-2
- Flavokawain C
Catalog No.:BCN8456
CAS No.:37308-75-1
- H-D-Tyr-OMe.HCl
Catalog No.:BCC3135
CAS No.:3728-20-9
- LY451395
Catalog No.:BCC5377
CAS No.:375345-95-2
- H-D-Ala(4-pyridyl)-OH.HCl
Catalog No.:BCC3325
CAS No.:37535-49-2
- 3-(2-Pyridyl)-Alanine
Catalog No.:BCC2656
CAS No.:37535-51-6
- H- Ala(2-pyridyl)-OH.2HCl
Catalog No.:BCC3318
CAS No.:37535-51-6 net
- 3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2655
CAS No.:37535-52-7
- 7'(Z)-(8''R,8'''R)-epi-salvianolic acid E
Catalog No.:BCC3319
CAS No.:
- Boc-Ala(4-pyridyl)-OH
Catalog No.:BCC3326
CAS No.:37535-57-2
- Boc-N-Me-Phe-OH
Catalog No.:BCC2615
CAS No.:37553-65-4
- Phorbol 12,13-dibutyrate
Catalog No.:BCC7870
CAS No.:37558-16-0
- Varenicline tartrate
Catalog No.:BCN2170
CAS No.:375815-87-5
- Furaltadone HCl
Catalog No.:BCC4662
CAS No.:3759-92-0
- Maraviroc
Catalog No.:BCC3675
CAS No.:376348-65-1
A novel ortho-dehalogenation reaction of 2-chlorocinnamic acid catalyzed by the pink yeast Rhodotorula rubra Y-1529.[Pubmed:10789983]
Chemosphere. 2000 Jun;40(12):1417-25.
In the present study, a resting cells suspension of Rhodotorula rubra Y-1529 was shown to have the capacity to perform an ortho-dehalogenation reaction on 2-Chlorocinnamic acid. The results from the biodegradation of U-[14C]benzoic acid, cinnamic acid, 3-chlorocinnamic acid and 4-chlorocinnamic acid suggest that the first step of the ortho-dehalogenation reaction occurred during the oxidation of the unsaturated C3 side chain of 2-Chlorocinnamic acid to 2-chlorobenzoic acid. None of the 2-chlorobenzoic acid was found in the biodegradation system, suggesting that this step was a highly regulated step. After the side-chain oxidation reaction, the hydroxylation of the benzene ring was determined to be at the para-position first, followed by the meta-position. The occurrence of 3:4-position ring fission reactions and the production of the final product, CO2, was proven by the biodegradation of U-[14C] benzoic acid. This oxidative dehalogenation reaction catalyzed by R. rubra was found to be regiospecific for 2-Chlorocinnamic acid; the chloride ion was probably removed after the ring fission reaction. A pathway of the ortho-dehalogenation reaction of 2-Chlorocinnamic acid catalyzed by R. rubra was proposed based on these data.
Inhibitory kinetics of chlorocinnamic acids on mushroom tyrosinase.[Pubmed:23958639]
J Biosci Bioeng. 2014 Feb;117(2):142-146.
Tyrosinase (EC 1.14.18.1) is the key enzyme of most food enzymatic oxidation. Tyrosinase inhibitors are important in food industry. In the present paper, 2-chlorcinnamic acid and 2,4-dichlorocinnamic acid were synthesized and the inhibitory kinetics on mushroom tyrosinase were investigated. The results showed that both compounds synthesized could inhibit tyrosinase activity. For monophenolase activity, both chlorocinnamic acids could extended the lag time and decrease the steady-state activities, 2-chlorcinnamic acid extended the lag time just by 5%, and 2,4-dichlorcinnamic acid extended the lag time more than by 30.4%. For diphenolase activity, the IC50 values of 2-chlorcinnamic acid and 2,4-dichlorocinnamic acid were determined to be 0.765 mM and 0.295 mM, respectively. The inhibition kinetics showed that 2-chlorcinnamic acid and 2,4-dichlorocinnamic acid displayed a reversible and uncompetitive mechanism. The inhibition constants were determined to be 0.348 mM and 0.159 mM, respectively. The research may supply the basis for designing new tyrosinase inhibitors.


