DHP LinkerCAS# 3749-36-8 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3749-36-8 | SDF | Download SDF |
| PubChem ID | 95559 | Appearance | Powder |
| Formula | C6H10O2 | M.Wt | 114.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
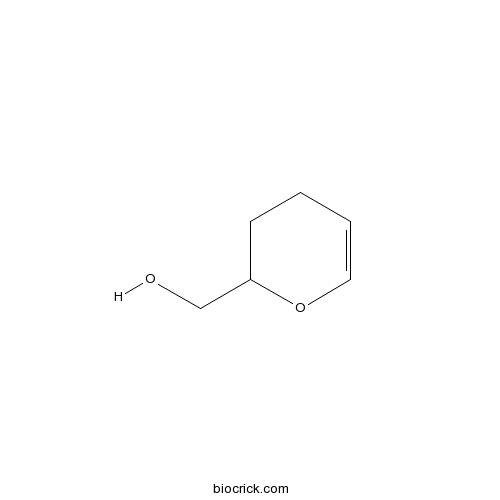
| Chemical Name | 3,4-dihydro-2H-pyran-2-ylmethanol | ||
| SMILES | C1CC(OC=C1)CO | ||
| Standard InChIKey | XMICBFRKICBBKD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H10O2/c7-5-6-3-1-2-4-8-6/h2,4,6-7H,1,3,5H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

DHP Linker Dilution Calculator

DHP Linker Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.7642 mL | 43.8212 mL | 87.6424 mL | 175.2848 mL | 219.106 mL |
| 5 mM | 1.7528 mL | 8.7642 mL | 17.5285 mL | 35.057 mL | 43.8212 mL |
| 10 mM | 0.8764 mL | 4.3821 mL | 8.7642 mL | 17.5285 mL | 21.9106 mL |
| 50 mM | 0.1753 mL | 0.8764 mL | 1.7528 mL | 3.5057 mL | 4.3821 mL |
| 100 mM | 0.0876 mL | 0.4382 mL | 0.8764 mL | 1.7528 mL | 2.1911 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DHP Linker
- Metastin (human)
Catalog No.:BCC5761
CAS No.:374683-24-6
- Kisspeptin 10 (human)
Catalog No.:BCC7415
CAS No.:374675-21-5
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- Boc-DL-Ala-OH
Catalog No.:BCC3050
CAS No.:3744-87-4
- DS2
Catalog No.:BCC7748
CAS No.:374084-31-8
- Cephaeline Hydrochloride
Catalog No.:BCC8307
CAS No.:3738-70-3
- Decloxizine
Catalog No.:BCC5529
CAS No.:3733-63-9
- 3-Quinuclidinone
Catalog No.:BCC8642
CAS No.:3731-38-2
- Flavokawain C
Catalog No.:BCN8456
CAS No.:37308-75-1
- H-D-Tyr-OMe.HCl
Catalog No.:BCC3135
CAS No.:3728-20-9
- Sennoside D
Catalog No.:BCN1005
CAS No.:37271-17-3
- Sennoside C
Catalog No.:BCN1004
CAS No.:37271-16-2
- Amikacin
Catalog No.:BCC5206
CAS No.:37517-28-5
- 2-Chlorocinnamic acid
Catalog No.:BCN5036
CAS No.:3752-25-8
- LY451395
Catalog No.:BCC5377
CAS No.:375345-95-2
- H-D-Ala(4-pyridyl)-OH.HCl
Catalog No.:BCC3325
CAS No.:37535-49-2
- 3-(2-Pyridyl)-Alanine
Catalog No.:BCC2656
CAS No.:37535-51-6
- H- Ala(2-pyridyl)-OH.2HCl
Catalog No.:BCC3318
CAS No.:37535-51-6 net
- 3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2655
CAS No.:37535-52-7
- 7'(Z)-(8''R,8'''R)-epi-salvianolic acid E
Catalog No.:BCC3319
CAS No.:
- Boc-Ala(4-pyridyl)-OH
Catalog No.:BCC3326
CAS No.:37535-57-2
- Boc-N-Me-Phe-OH
Catalog No.:BCC2615
CAS No.:37553-65-4
- Phorbol 12,13-dibutyrate
Catalog No.:BCC7870
CAS No.:37558-16-0
- Varenicline tartrate
Catalog No.:BCN2170
CAS No.:375815-87-5
[Molecular and pharmacological bases for the gating regulation of L-type voltage-dependent Ca2+ channels].[Pubmed:14993732]
Nihon Yakurigaku Zasshi. 2004 Mar;123(3):197-209.
The voltage-dependent L-type Ca(2+) channel plays a key role in the spacial and temporal regulation of Ca(2+). In cardiac excitation-contraction coupling, Ca(2+)-induced Ca(2+) release (CICR) from ryanodine receptors (RyRs), triggered by Ca(2+) entry through the nearby L-type Ca(2+) channel, induces the Ca(2+)-dependent inactivation (CDI) of the Ca(2+) channel. We demonstrated that the CICR-dependent CDI of L-type Ca(2+) channels, under control of the privileged cross-signaling between L-type Ca(2+) channels and RyRs, plays important roles for monitoring and tuning the SR Ca(2+) content via changes of AP waveform and the amount of Ca(2+)-influx during AP in ventricular myocytes. L-type Ca(2+) channels are modulated by the binding of Ca(2+) channel antagonists and agonists to the pore-forming alpha(1C) subunit. We identified Phe(1112) and Ser(1115) in the pore-forming IIIS5-S6 linker region of the alpha(1C) subunit as critical determinants of the binding of dihydropyridines (DHP). Interestingly, double mutant Ca(2+) channel (F1112A/S1115A) failed to discriminate between a DHP Ca(2+) channel agonist and antagonist stereoisomers. We proposed that Phe(1112) and Ser(1115) in the pore-forming IIIS5-S6 linker region is required for the stabilization of the Ca(2+) channel in the open state by Ca(2+) channel agonists and further proposed a novel model for the DHP-binding pocket of the alpha(1C) subunit. These integrative studies on the gating regulation of cardiac L-type Ca(2+) channels will provide the molecular basis for the pharmacology of Ca(2+) channel modulators.
2,3-Dihydroxypyridine-loaded cellulose: a new macromolecular chelator for metal enrichment prior to their determination by atomic absorption spectrometry.[Pubmed:12904958]
Anal Bioanal Chem. 2003 Nov;377(6):1079-86.
New macromolecular chelators have been synthesized, by loading 2,3-dihydroxypyridine (DHP) on cellulose via linkers -NH-CH(2)-CH(2)-NH-SO(2)-C(6)H(4)-N=N- and -SO(2)-C(6)H(4)-N=N-, and characterized by elemental analysis, TGA, IR, and CPMAS (13)C NMR spectra. The cellulose with DHP anchored by the shorter linker had better sorption capacity (between 69.7 and 431.1 micromol g(-1)) for Co(II), Ni(II), Cu(II), Zn(II), Cd(II), Pb(II), and Fe(III)) than the other (51.9-378.1 micromol g(-1)); the former was therefore studied in detail as a solid extractant for these metal ions. The optimum pH ranges for quantitative sorption (recovery 97.6-99.8%) on this matrix were: 7.0-9.0, 6.0-9.0, 3.0-8.0, 6.0-8.0, 6.0-9.0, 6.0-7.0, and 2.0-6.0 respectively. Desorption was quantitative with 0.5 mol L(-1) HCl and 0.5 mol L(-1) HNO(3) (for Pb). Simultaneous sorption (at pH 7.0) of all metal ions other than Fe(III) was possible if their total concentration did not exceed the sorption capacity (lowest value). The recovery of seven metal ions from their mixture at pH 6.0 was nearly quantitative when the concentration level of each metal ion was 0.2 microg mL(-1). The optimum flow rate of metal ion solutions for quantitative sorption of metal onto a column packed with DHP-modified cellulose was 2-7 mL min(-1), whereas for desorption the optimum flow rate for the acid solution was 2-4 mL min(-1). The time needed to reach 50% of the total loading capacity ( t(1/2)) was <5 min for all the metal ions except Ni and Pb. The limit of detection (blank+3 s) was from 0.70 to 4.75 microg L(-1) and the limit of quantification (blank+10 s) was between 0.79 and 4.86 microg L(-1). The tolerance limits for NaCl, NaBr, NaI, NaNO(3), Na(2)SO(4), Na(3)PO(4,) humic acid, EDTA, Ca(II), and Mg(II) for sorption of all metal ions are reported. The column packed with DHP-anchored cellulose can be reused at least 20 times for enrichment of metal ions in water sample. It has been used to enrich all the metal ions in pharmaceutical and water samples before their determination by flame AAS. RSD for these determinations was between 1.1 and 6.9%.
Two mechanistically distinct effects of dihydropyridine nifedipine on CaV1.2 L-type Ca(2)(+) channels revealed by Timothy syndrome mutation.[Pubmed:22554770]
Eur J Pharmacol. 2012 Jun 15;685(1-3):15-23.
Dihydropyridine Ca(2+) channel antagonists (DHPs) block Ca(V)1.2 L-type Ca(2+) channels (LTCCs) by stabilizing their voltage-dependent inactivation (VDI); however, it is still not clear how DHPs allosterically interact with the kinetically distinct (fast and slow) VDI. Thus, we analyzed the effect of a prototypical DHP, nifedipine on LTCCs with or without the Timothy syndrome mutation that resides in the I-II linker (L(I)-(II)) of Ca(V)1.2 subunits and impairs VDI. Whole-cell Ba(2+) currents mediated by rabbit Ca(V)1.2 with or without the Timothy mutation (G436R) (analogous to the human G406R mutation) were analyzed in the presence and absence of nifedipine. In the absence of nifedipine, the mutation significantly impaired fast closed- and open-state VDI (CSI and OSI) at -40 and 0 mV, respectively, but did not affect channels' kinetics at -100 mV. Nifedipine equipotently blocked these channels at -80 mV. In wild-type LTCCs, nifedipine promoted fast CSI and OSI at -40 and 0 mV and promoted or stabilized slow CSI at -40 and -100 mV, respectively. In LTCCs with the mutation, nifedipine resumed the impaired fast CSI and OSI at -40 and 0 mV, respectively, and had the same effect on slow CSI as in wild-type LTCCs. Therefore, nifedipine has two mechanistically distinct effects on LTCCs: the promotion of fast CSI/OSI caused by L(I-II) at potentials positive to the sub-threshold potential and the promotion or stabilization of slow CSI caused by different mechanisms at potentials negative to the sub-threshold potential.
Biologically active recombinant carp LH as a spawning-inducing agent for carp.[Pubmed:27999090]
J Endocrinol. 2017 Mar;232(3):391-402.
Currently, spawning is induced in carp species by carp pituitary extract (CPE) and a combination of synthetic agonist of GnRH combined with a dopamine antagonist. The main goal of this study was the production of recombinant gonadotropins (GtHs) on a large scale to serve as an alternative to currently used agents. We produced carp (c) recombinant (r) Lh as a single chain in the methylotrophic yeast Pichia pastoris Lha subunit was joined with Lhb subunit with a flexible linker of three glycine-serine repeats and six Histidines to form a mature protein, the beta-subunit formed the N-terminal part and the alpha-subunit formed the C-terminal part. The ability of the rcLh to elicit biological response was tested by in vivo stimulation of estradiol (E2) and 17alpha,20beta-dihydroxy-4-pregnen-3-one (DHP) and by its in vivo potency to induce ovulation and spawning induction. rcLh tested in this work significantly enhanced both E2 and DHP secretion in a dose-dependent manner similar to the results obtained with CPE. E2 levels showed a moderate rise following the priming injection and a subsequent decrease during the rest of the trial. DHP levels were only increased after the resolving injection, approximately 5 h before spawning. At the highest dose of rcLh (350 microg/kg BW), the recombinant protein was more efficient than CPE in terms of both spawning success and fertilization rate. It is shown here that rcLh can elicit the secretion of DHP in vivo and actually trigger spawning. These novel findings introduce the potential of utilizing recombinant gonadotropins in aquaculture.
Key roles of Phe1112 and Ser1115 in the pore-forming IIIS5-S6 linker of L-type Ca2+ channel alpha1C subunit (CaV 1.2) in binding of dihydropyridines and action of Ca2+ channel agonists.[Pubmed:12869628]
Mol Pharmacol. 2003 Aug;64(2):235-48.
Voltage-dependent L-type Ca2+ channels are modulated by the binding of Ca2+ channel antagonists and agonists to the pore-forming alpha1c subunit (CaV 1.2). We recently identified Ser1115 in IIIS5-S6 linker of alpha1C subunit as a critical determinant of the action of 1,4-dihydropyridine agonists. In this study, we applied alanine-scanning mutational analysis in IIIS5-S6 linker of rat brain alpha1C subunit (rbCII) to illustrate the role of pore-forming IIIS5-S6 linker in the action of Ca2+ channel modulators. Ca2+ channel currents through wild-type (rbCII) or mutated alpha1C subunits, transiently expressed in BHK6 cells with beta1a and alpha2/delta subunits, were analyzed. The replacement of Phe1112 by Ala (F1112A) significantly impaired the sensitivity to Ca2+ channel agonists (S)-(-)-Bay k 8644 and FPL-64176, and modestly to 1,4-dihydropyridine (DHP) antagonists. The low sensitivity of F1112A and S1115A to DHP antagonists was consistent with the reduced binding affinity for [3H](+)PN200-110. The replacement of Phe1112 by Tyr, but not by Ala, restored the long openings produced by FPL-64176, thus indicating the critical role of aromatic ring of Phe1112 in the Ca2+ channel agonist action. Interestingly, double-mutant Ca2+ channel (F1112A/S1115A) failed to discriminate between Ca2+ channel agonist (S)-(-)-1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-[trifluoromethyl] phenyl)-3-pyridine carboxylic acid methyl ester (Bay k 8644) and antagonist (R)-(+)-Bay k 8644 and was blocked by the two enantiomers in an identical manner. These results indicate that both Phe1112 and Ser1115 in linker IIIS5-S6 are required for the action of Ca2+ channel agonists. A model of the DHP receptor is proposed to visualize possible interactions of Phe1112, Ser1115, and other DHP-sensing residues with a typical DHP ligand nifedipine.


