3',4',7-TrimethoxyquercetinCAS# 6068-80-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6068-80-0 | SDF | Download SDF |
| PubChem ID | 5748558.0 | Appearance | Powder |
| Formula | C18H16O7 | M.Wt | 344.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
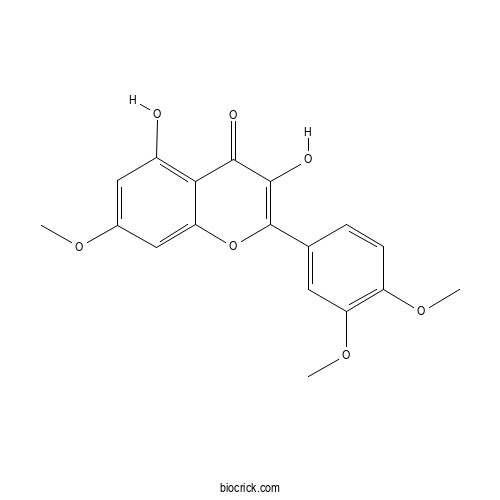
| Chemical Name | 2-(3,4-dimethoxyphenyl)-3,5-dihydroxy-7-methoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)OC)O)O)OC | ||
| Standard InChIKey | OEEUHNAUMMATJT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O7/c1-22-10-7-11(19)15-14(8-10)25-18(17(21)16(15)20)9-4-5-12(23-2)13(6-9)24-3/h4-8,19,21H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3',4',7-Trimethoxyquercetin Dilution Calculator

3',4',7-Trimethoxyquercetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9043 mL | 14.5214 mL | 29.0428 mL | 58.0855 mL | 72.6069 mL |
| 5 mM | 0.5809 mL | 2.9043 mL | 5.8086 mL | 11.6171 mL | 14.5214 mL |
| 10 mM | 0.2904 mL | 1.4521 mL | 2.9043 mL | 5.8086 mL | 7.2607 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5809 mL | 1.1617 mL | 1.4521 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5809 mL | 0.7261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anthranoyllycoctonine
Catalog No.:BCX1319
CAS No.:22413-78-1
- Ethyl ganoderate A
Catalog No.:BCX1318
CAS No.:2242593-18-4
- Ethyl ganoderenate D
Catalog No.:BCX1317
CAS No.:2226858-23-5
- Tunicoidine A
Catalog No.:BCX1316
CAS No.:1415979-39-3
- Rabdosia acid A
Catalog No.:BCX1315
CAS No.:1884697-13-5
- Neoartanin
Catalog No.:BCX1314
CAS No.:104196-69-2
- Poricoic acid BM
Catalog No.:BCX1313
CAS No.:1815623-74-5
- Isooxypeucedanin
Catalog No.:BCX1312
CAS No.:5058-15-1
- Oxypeucedanin hydrate-3”-ethyl ether
Catalog No.:BCX1311
CAS No.:55481-87-3
- Theasaponin E2
Catalog No.:BCX1310
CAS No.:220114-30-7
- 3'-O-Angeloylhamaudol
Catalog No.:BCX1309
CAS No.:84272-84-4
- Entadamide A
Catalog No.:BCX1308
CAS No.:100477-88-1
- Wushanicaritin
Catalog No.:BCX1321
CAS No.:521-45-9
- 5-MethoxyPinocembroside
Catalog No.:BCX1322
CAS No.:1450878-89-3
- Decuroside V
Catalog No.:BCX1323
CAS No.:96648-59-8
- Anhydronotoptol
Catalog No.:BCX1324
CAS No.:88206-51-3
- Lycaconitine
Catalog No.:BCX1325
CAS No.:25867-19-0
- Methyllycaconitine
Catalog No.:BCX1326
CAS No.:21019-30-7
- 6-Methylrhein
Catalog No.:BCX1327
CAS No.:401621-27-0
- Multiflorin B
Catalog No.:BCX1328
CAS No.:52657-01-9
- Multiflorin A
Catalog No.:BCX1329
CAS No.:1350028-90-8
- Quinquenoside III
Catalog No.:BCX1330
CAS No.:208764-53-8
- Pseudoginsenoside RC1
Catalog No.:BCX1331
CAS No.:102805-32-3
- β-D-Glucopyranoside, (3β,12β)-20-[(6-O-α-L-arabinofuranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-, 6-acetate
Catalog No.:BCX1332
CAS No.:1613477-95-4
A high-speed counter-current chromatography- HPLC-DAD method for preparative isolation and purification of two polymethoxylated flavones from Taraxacum mongolicum.[Pubmed:19476701]
J Chromatogr Sci. 2009 May-Jun;47(5):349-53.
After an initial clean-up step on silica gel, a preparative high-speed counter-current chromatography coupled with on-line high-performance liquid chromatography-diode array detection method (HSCCC-HPLC-DAD) was successfully developed for the isolation and determination two polymethoxylated flavones, 3',4',7-trimethoxyquercetin and artemetin, from the aerial part of Taraxacum mongolicum. The HSCCC separation was performed with a two-phase solvent system composed of n-hexane-ethyl acetate-methanol-water (6:5:6:5, v/v/v/v) at a flow rate of 1.5 mL/min and at 800 rpm. The on-line purity monitoring of a representative aliquot from each HSCCC fraction was operated automatically. Using this method, fractions with high purity were collected. The HSCCC purification step was done in 5 h, and afforded 84.2 mg of 3',4',7-trimethoxyquercetin and 52.3 mg of artemetin, with purity over 98% from 200 mg of the enriched extracts of T. mongolicum. The structures were identified by electorspray ionization mass spectrometry and 1H NMR experiments. To our best knowledge, 3',4',7-trimethoxyquercetin was obtained from the plant of genus Taraxacum for the first time by our group. This hyphenated method could be used for the preparation of bioactive compounds with higher purity from natural products.
Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target.[Pubmed:7923555]
Cancer Chemother Pharmacol. 1994;34(6):459-64.
This study demonstrates that the flavonoid quercetin (Q), a plant-derived compound with low toxicity in vivo, greatly potentiates the growth-inhibitory activity of Adriamycin (ADR) on MCF-7 ADR-resistant human breast cancer cells. The effect of Q was dose-dependent at concentrations ranging between 1 and 10 microM. Since ADR resistance in these cells is associated with the expression of high levels of P-glycoprotein (Pgp), we evaluated the effect of Q and related flavonoids of Pgp activity in cytofluorographic efflux experiments with the fluorescent dye rhodamine 123 (Rh 123). Our results indicate that Q and 3-OMe Q (3',4',7-trimethoxyquercetin) but not the 3-rhamnosylglucoside of Q (rutin) inhibit the Pgp pump-efflux activity in a dose-related manner. Moreover, 10 microM Q reduces the expression of the immunoreactive Pgp in MCF-7 ADR-resistant cells as evaluated by cytofluorimetric assay. In conclusion, these findings provide a further biological basis for the potential therapeutic application of Q as an anti-cancer drug either alone or in combination with ADR in multidrug-resistant breast tumor cells.


