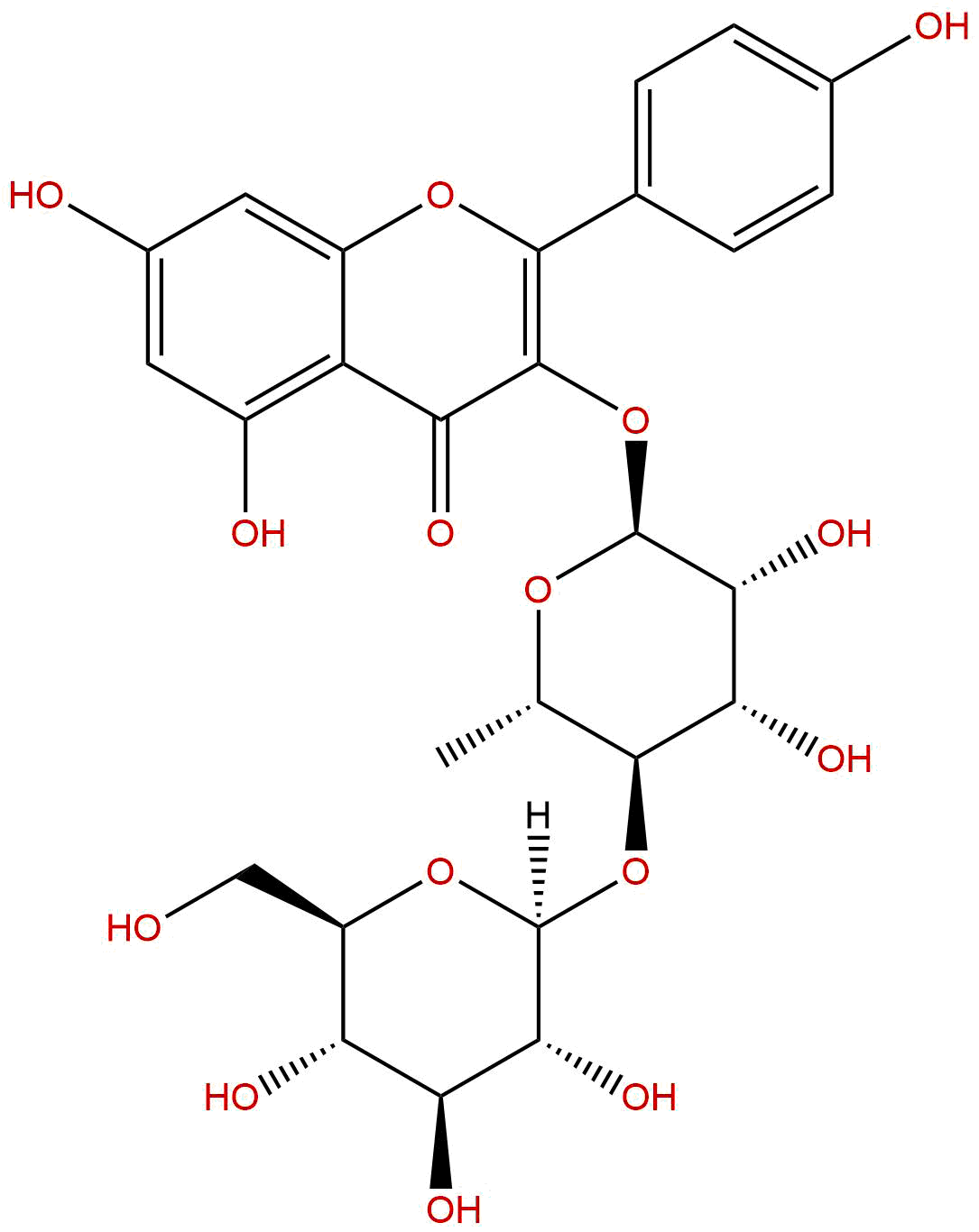
Multiflorin BCAS# 52657-01-9 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 52657-01-9 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C27H30O15 | M.Wt | 594.52 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Multiflorin B Dilution Calculator

Multiflorin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.682 mL | 8.4101 mL | 16.8203 mL | 33.6406 mL | 42.0507 mL |
| 5 mM | 0.3364 mL | 1.682 mL | 3.3641 mL | 6.7281 mL | 8.4101 mL |
| 10 mM | 0.1682 mL | 0.841 mL | 1.682 mL | 3.3641 mL | 4.2051 mL |
| 50 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6728 mL | 0.841 mL |
| 100 mM | 0.0168 mL | 0.0841 mL | 0.1682 mL | 0.3364 mL | 0.4205 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Methylrhein
Catalog No.:BCX1327
CAS No.:401621-27-0
- Methyllycaconitine
Catalog No.:BCX1326
CAS No.:21019-30-7
- Lycaconitine
Catalog No.:BCX1325
CAS No.:25867-19-0
- Anhydronotoptol
Catalog No.:BCX1324
CAS No.:88206-51-3
- Decuroside V
Catalog No.:BCX1323
CAS No.:96648-59-8
- 5-MethoxyPinocembroside
Catalog No.:BCX1322
CAS No.:1450878-89-3
- Wushanicaritin
Catalog No.:BCX1321
CAS No.:521-45-9
- 3',4',7-Trimethoxyquercetin
Catalog No.:BCX1320
CAS No.:6068-80-0
- Anthranoyllycoctonine
Catalog No.:BCX1319
CAS No.:22413-78-1
- Ethyl ganoderate A
Catalog No.:BCX1318
CAS No.:2242593-18-4
- Ethyl ganoderenate D
Catalog No.:BCX1317
CAS No.:2226858-23-5
- Tunicoidine A
Catalog No.:BCX1316
CAS No.:1415979-39-3
- Multiflorin A
Catalog No.:BCX1329
CAS No.:1350028-90-8
- Quinquenoside III
Catalog No.:BCX1330
CAS No.:208764-53-8
- Pseudoginsenoside RC1
Catalog No.:BCX1331
CAS No.:102805-32-3
- β-D-Glucopyranoside, (3β,12β)-20-[(6-O-α-L-arabinofuranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-, 6-acetate
Catalog No.:BCX1332
CAS No.:1613477-95-4
- Pseudoginsenoside F8
Catalog No.:BCX1333
CAS No.:69884-01-1
- Ajuforrestin B
Catalog No.:BCX1334
CAS No.:708277-48-9
- Ilexoside XLVIII
Catalog No.:BCX1335
CAS No.:129095-76-7
- Gosferol
Catalog No.:BCX1336
CAS No.:37551-62-5
- rel-(+)-(1R,2Z,7Z,10S,11S)-10-(Acetyloxy)-7,12,12-trimethylbicyclo[9.1.0]dodeca-2,7-dien-4-one
Catalog No.:BCX1337
CAS No.:886439-01-6
- Phloyoside I
Catalog No.:BCX1338
CAS No.:139757-58-7
- Dracaenoside F
Catalog No.:BCX1339
CAS No.:109460-83-5
- Linderanine C
Catalog No.:BCX1340
CAS No.:139681-96-2
Camellia nitidissima Chi leaf as pancreatic lipase inhibitors: Inhibition potentials and mechanism.[Pubmed:34231229]
J Food Biochem. 2021 Sep;45(9):e13837.
In this study, Camellia nitidissima Chi leaf extract was investigated for its compounds and pancreatic lipase inhibitory potentials. The interaction was determined using ultraviolet (UV) spectroscopy, circular dichroism (CD), fluorescence spectroscopy (FS), and molecular docking to understand the inhibiton, kinetic, and conformation of extraction-pancreatic lipase complex. C. nitidissima Chi leaf extraction inhibited the pancreatic lipase activity in a dose-dependent manner at the concentration of 1-12 mg/ml. The Lineweaver-Burk plots indicated that the inhibition on pancreatic lipase by extraction was noncompetitive. In addition, the decrease in alpha-helix contents, increase in beta-sheet and beta-turn, and decrease in fluorescence intensity after extraction treatment indicated that the conformation of pancreatic lipase was changed. This work revealed that C. nitidissima Chi leaf extraction played a significant role in inhibiting pancreatic lipase activity and brought out a solution of delay fat accumulation. PRACTICAL APPLICATIONS: This study reports the components in the extract of C. nitidissima Chi leaf and its inhibitory effect and mechanism of pancreatic lipase. C. nitidissima Chi leaf is a good source of bioactive components, including Multiflorin B, kaempferol-3-O-rutinoside, vicenin-2, apigenin-6-C-pentosyl-8-C-hexosyl, vitexin, kaempferol, and other ingredients. It can inhibit pancreatic lipase and be used to control obesity and treat hyperlipidemia. This study also revealed the structure changes of C. nitidissima Chi leaf extract on pancreatic lipase, and further revealed the inhibitory mechanism of C. nitidissima Chi leaf extract on lipase, which provides a theoretical basis for C. nitidissima Chi leaf as a lipase inhibitor.
Phytochemical characterization of Rosa multiflora Thunb. (Rosaceae) in Japan and South Korea, with a focus on the bioactive flavonol glycoside 'multiflorin A'.[Pubmed:30949951]
J Nat Med. 2019 Jun;73(3):555-565.
Dried achene or anthocarpous accessory fruits of Rosa multiflora Thunb., Rosae fructus ("Eijitsu" in Japanese), have been used in clinical practice to improve constipation within traditional Japanese medicine. Recently, it has been claimed that the efficacy of this crude drug is decreasing, and multiflorin A, the purgative component, was not detected within the tested samples. In order to clarify the causes of this issue, we investigated Rosa section Synstylae (Rosaceae), including R. multiflora, growing in Japan and South Korea with a focus on the secondary metabolite, multiflorin A. We recognize that there are two chemotypes based on the presence (Type I) or absence (Type II) of multiflorin A. Type I contains quercitrin, multinoside A, Multiflorin B, and multinoside A acetate as major index compounds. Type II contains hyperin, isoquercitrin, quercetin 3-O-glucuronide, and 3'-methoxy-isoquercitrin as the major index compounds. The chemotype of Rosa section Synstylae (Rosaceae) plants collected in Japan (excluding Tsushima Island) were all classified as Type I with exception of two species, R. luciae and R. sambucina. On the other hand, both Type I and Type II were detected within Rosae fructus obtained from R. multiflora collected in South Korea and Tsushima Island, Japan. The results indicate that Rosae fructus from R. multiflora (Type I) from Japan, excluding Tsushima Island, should be employed clinically, which we describe as purgative.
The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of Rhodiola sachalinensis A. BOR.[Pubmed:23018923]
Molecules. 2012 Sep 27;17(10):11484-94.
Isolation of compounds from the root of Rhodiola sachalinensis (RRS) yielded tyrosol (1), salidroside (2), Multiflorin B (3), kaempferol-3,4'-di-O-beta-D-glucopyranoside (4), afzelin (5), kaempferol (6), rhodionin (7), and rhodiosin (8). Quantification of these compounds was performed by high-performance liquid chromatography (HPLC). To investigate the antioxidant and anti-inflammatory effects of the compounds, DPPH radical scavenging, NBT superoxide scavenging and nitric oxide production inhibitory activities were examined in LPS-stimulated Raw 264.7 cells. We suggest that the major active components of RRS are herbacetin glycosides, exhibiting antioxidant activity, and kaempferol, exhibiting anti-inflammatory activity.
Peach leaf contains multiflorin a as a potent inhibitor of glucose absorption in the small intestine in mice.[Pubmed:22863923]
Biol Pharm Bull. 2012;35(8):1264-8.
Peach leaf extract has anti-hyperglycemic effects on the postprandial blood glucose level in glucose-loaded mice. In our previous study, the mechanism of action was considered to be the inhibition of glucose absorption in the small intestine. To elucidate the active principle in peach leaf, purification of the active compound and a structure determination were performed. With the use of bioassay-guided fractionation using glucose-loaded mice, the acetylated kaempferol glycoside multiflorin A (MFA), a potent inhibitor of glucose absorption from the intestine, was isolated from the MeOH extract of leaf of the edible peach Prunus persica. The structure was identified by HPLC using thiazolizine derivatives and by an analysis of its spectral data. The inhibitory effect of MFA against glucose absorption was demonstrated in the dose dependent manner in mice. However, as the deacetylated analog of MFA, Multiflorin B did not show the activity at the in vivo, the activity of MFA was suggested to depend on the acetyl group on the sugar moiety. This is the first report of anti-hyperglycemic activity of MFA in peach leaf extract. MFA may be useful in functional foods or medicines for preventing the postprandial absorption of glucose in hyperglycemia.
Isolation and evaluation of kaempferol glycosides from the fern Neocheiropteris palmatopedata.[Pubmed:20100622]
Phytochemistry. 2010 Apr;71(5-6):641-7.
Kaempferol glycosides, named palmatosides A (1), B (2) and C (3), together with three known kaempferol glycosides, multiflorins A (4) and B (5), and afzelin (6), were isolated from the roots of the fern Neocheiropteris palmatopedata. Palmatosides A (1) and B (2) each possessed an unusual sugar moiety containing a 4,4-dimethyl-3-oxo-butoxy substituent group. The isolated compounds were evaluated for their cancer chemopreventive potential based on their ability to inhibit tumor necrosis factor alpha (TNF-alpha)-induced NF-kappaB activity, nitric oxide (NO) production, aromatase, quinone reductase 2 (QR2) and COX-1/-2 activities. Palmatosides B (2) and C (3) inhibited TNF-alpha-induced NF-kappaB activity with IC(50) values of 15.7 and 24.1 microM, respectively; multiflorin A (4) inhibited aromatase enzyme with an IC(50) value of 15.5 microM; afzelin (6) showed 68.3% inhibition against QR2 at a concentration of 11.5 microg/ml; palmatoside A (1) showed 52% inhibition against COX-1 enzyme at a concentration of 10 microg/ml; and Multiflorin B (5) showed 52% inhibition against nitric oxide production at a concentration of 20 microg/ml. In addition, compounds 3-6 were shown to bind QR2 enzyme using LC-MS ultrafiltration binding assay.
Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg.[Pubmed:18449498]
Arch Pharm Res. 2008 Apr;31(4):424-8.
Chemical investigation of the 80% Me(2)CO extract from the seeds of Prunus tomentosa led to the isolation and identification of six flavonoids: kaempferol (1), kaempferol 3-O-alpha-L-rhamnopyranoside (2; afzelin), kaempferol 3-O-beta-D-(6-acetyl)-glucopyranosyl(1-->4)-alpha-L-rhamnopyranoside (3; multiflorin A), kaempferol 3-O-beta-D-glucopyranosyl(1-->4)-alpha-L-rhamnopyranoside (4; Multiflorin B), quercetin 3-O-alpha-L-rhamnopyranoside (5; quercitrin), and quercetin 3-O-beta-D-glucopyranosyl (1-->4)-alpha-L-rhamnopyranoside (6; multinoside A). Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E(2) production in interferon-gamma (INF-gamma) and lipopolysaccharide (LPS)-activated RAW 264.7 cells in vitro (COX-2) of the isolated compounds were evaluated. Compounds 1, 5, and 6 exhibited potent anti-oxidative activity in the DPPH radical scavenging assay with IC(50) values of 57.2, 59.4, and 54.3 microg/mL respectively. The positive control, ascorbic acid, had an IC(50) of 55.5 mug/mL. Compounds 1, 5, and 6 also reduced COX-2 levels in a dose dependent manner with IC(50) values of 10.2, 8.7, and 9.6 microg/mL respectively, with the positive control, indomethacin, having an IC(50) of 5.1 microg/mL. All six compounds inhibited NO production in a dose dependent manner with IC(50) values of 35.1, 42.8, 40.0, 44.8, 43.7, and 43.9 microg/mL respectively, while the positive control, L-NMMA, had an IC(50) of 42.1 microg/mL.
The extract of the flowers of Prunus persica, a new cosmetic ingredient, protects against solar ultraviolet-induced skin damage in vivo.[Pubmed:11917253]
J Cosmet Sci. 2002 Jan-Feb;53(1):27-34.
The flowers of Prunus persica Batsch have been used for skin disorders in East Asia from ancient times. In this investigation, the ethanol extract from this plant material was prepared and several major constituents were isolated. In addition, the protective effects of the extract were evaluated against solar ultraviolet (UV)-induced skin damage using in vivo animal models of UVB-induced erythema in guinea pigs and ear edema in ICR mice. From the extract, four kaempferol glycoside derivatives were successfully isolated and their contents were measured with HPLC. Among the derivatives isolated, the content of Multiflorin B was highest (3.3%, w/w). The P. persica extract clearly inhibited UVB-induced erythema formation dose dependently when topically applied (IC(50) = 0.5 mg/cm(2)). It also inhibited UVB-induced ear edema (49% inhibition at 3.0 mg/ear). Moreover, Multiflorin B inhibited UVB-induced erythema formation (80% inhibition at 0.3 mg/cm(2)), indicating that this compound is one of the active principles of the extract. All these results suggest that P. persica extract may be useful for protection against UVB-induced skin damage when topically applied.


