Multiflorin ACAS# 1350028-90-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1350028-90-8 | SDF | Download SDF |
| PubChem ID | 5319939.0 | Appearance | Powder |
| Formula | C29H32O16 | M.Wt | 636.56 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
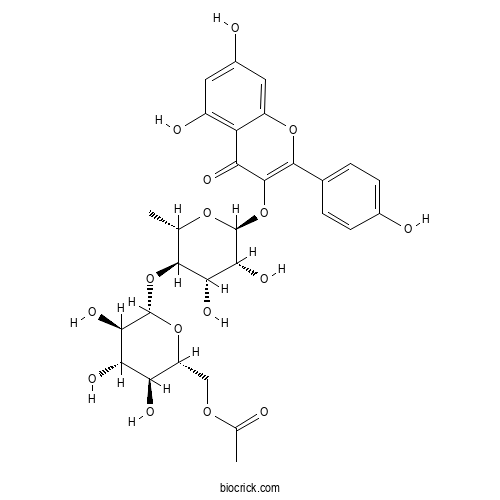
| Chemical Name | [(2R,3S,4S,5R,6S)-6-[(2S,3R,4S,5R,6S)-6-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-3-yl]oxy-4,5-dihydroxy-2-methyloxan-3-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methyl acetate | ||
| SMILES | CC1C(C(C(C(O1)OC2=C(OC3=CC(=CC(=C3C2=O)O)O)C4=CC=C(C=C4)O)O)O)OC5C(C(C(C(O5)COC(=O)C)O)O)O | ||
| Standard InChIKey | KXOPSQZLBRPJGX-KEBUVGJQSA-N | ||
| Standard InChI | InChI=1S/C29H32O16/c1-10-25(44-29-23(38)21(36)19(34)17(43-29)9-40-11(2)30)22(37)24(39)28(41-10)45-27-20(35)18-15(33)7-14(32)8-16(18)42-26(27)12-3-5-13(31)6-4-12/h3-8,10,17,19,21-25,28-29,31-34,36-39H,9H2,1-2H3/t10-,17+,19+,21-,22-,23+,24+,25-,28-,29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Multiflorin A Dilution Calculator

Multiflorin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5709 mL | 7.8547 mL | 15.7094 mL | 31.4189 mL | 39.2736 mL |
| 5 mM | 0.3142 mL | 1.5709 mL | 3.1419 mL | 6.2838 mL | 7.8547 mL |
| 10 mM | 0.1571 mL | 0.7855 mL | 1.5709 mL | 3.1419 mL | 3.9274 mL |
| 50 mM | 0.0314 mL | 0.1571 mL | 0.3142 mL | 0.6284 mL | 0.7855 mL |
| 100 mM | 0.0157 mL | 0.0785 mL | 0.1571 mL | 0.3142 mL | 0.3927 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Multiflorin B
Catalog No.:BCX1328
CAS No.:52657-01-9
- 6-Methylrhein
Catalog No.:BCX1327
CAS No.:401621-27-0
- Methyllycaconitine
Catalog No.:BCX1326
CAS No.:21019-30-7
- Lycaconitine
Catalog No.:BCX1325
CAS No.:25867-19-0
- Anhydronotoptol
Catalog No.:BCX1324
CAS No.:88206-51-3
- Decuroside V
Catalog No.:BCX1323
CAS No.:96648-59-8
- 5-MethoxyPinocembroside
Catalog No.:BCX1322
CAS No.:1450878-89-3
- Wushanicaritin
Catalog No.:BCX1321
CAS No.:521-45-9
- 3',4',7-Trimethoxyquercetin
Catalog No.:BCX1320
CAS No.:6068-80-0
- Anthranoyllycoctonine
Catalog No.:BCX1319
CAS No.:22413-78-1
- Ethyl ganoderate A
Catalog No.:BCX1318
CAS No.:2242593-18-4
- Ethyl ganoderenate D
Catalog No.:BCX1317
CAS No.:2226858-23-5
- Quinquenoside III
Catalog No.:BCX1330
CAS No.:208764-53-8
- Pseudoginsenoside RC1
Catalog No.:BCX1331
CAS No.:102805-32-3
- β-D-Glucopyranoside, (3β,12β)-20-[(6-O-α-L-arabinofuranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-, 6-acetate
Catalog No.:BCX1332
CAS No.:1613477-95-4
- Pseudoginsenoside F8
Catalog No.:BCX1333
CAS No.:69884-01-1
- Ajuforrestin B
Catalog No.:BCX1334
CAS No.:708277-48-9
- Ilexoside XLVIII
Catalog No.:BCX1335
CAS No.:129095-76-7
- Gosferol
Catalog No.:BCX1336
CAS No.:37551-62-5
- rel-(+)-(1R,2Z,7Z,10S,11S)-10-(Acetyloxy)-7,12,12-trimethylbicyclo[9.1.0]dodeca-2,7-dien-4-one
Catalog No.:BCX1337
CAS No.:886439-01-6
- Phloyoside I
Catalog No.:BCX1338
CAS No.:139757-58-7
- Dracaenoside F
Catalog No.:BCX1339
CAS No.:109460-83-5
- Linderanine C
Catalog No.:BCX1340
CAS No.:139681-96-2
- Paratocarpin K
Catalog No.:BCX1341
CAS No.:170900-13-7
A novel purgative mechanism of multiflorin A involves changing intestinal glucose absorption and permeability.[Pubmed:37054485]
Phytomedicine. 2023 Jun;114:154805.
BACKGROUND: Multiflorin A (MA) is a potential active ingredient of traditional herbal laxative, Pruni semen, with unusual purgative activity and an unclear mechanism, and inhibiting intestinal glucose absorption is a promising mechanism of novel laxatives. However, this mechanism still lacks support and a description of basic research. PURPOSE: This study aimed to determine the main contribution of MA to the purgative activity of Pruni semen and elucidate the effect intensity, characteristics, site, and mechanism of MA in mice, and determine the novel mechanism of traditional herbal laxatives from the perspective of intestinal glucose absorption. METHODS: We induced diarrhoea in mice by administering Pruni semen and MA, and the defecation behaviour, glucose tolerance, and intestinal metabolism were analysed. The effects of MA and its metabolite on peristalsis of the intestinal smooth muscle were evaluated using an intestinal motility assay in vitro. Intestinal tight junction proteins, aquaporins, and glucose transporters expression were analysed using immunofluorescence; gut microbiota and faecal metabolites were analysed using 16S rRNA and liquid chromatography-mass spectrometry. RESULTS: MA administration (20 mg/kg) induced watery diarrhoea in over half of the experimental mice. The activity of MA in lowering peak postprandial glucose levels was synchronous with purgative action, with the acetyl group being the active moiety. MA was metabolised primarily in the small intestine, where it decreased sodium-glucose cotransporter-1, occludin, and claudin1 expression, then inhibited glucose absorption, resulting in a hyperosmotic environment. MA also increased the aquaporin3 expression to promote water secretion. Unabsorbed glucose reshapes the gut microbiota and their metabolism in the large intestine and the increasing gas and organic acid promoted defecation. After recovery, the intestinal permeability and glucose absorption function returned, and the abundance of probiotics such as Bifidobacterium increased. CONCLUSION: The purgative mechanism of MA involves inhibiting glucose absorption, altering permeability and water channels to promote water secretion in the small intestine, and regulating gut microbiota metabolism in the large intestine. This study is the first systematic experimental study on the purgative effect of MA. Our findings provide new insight into the study of novel purgative mechanisms.
Evaluation of the chemical pro fi le from four germplasms sources of Pruni Semen using UHPLC-LTQ-Orbitrap-MS and multivariate analyses.[Pubmed:36320598]
J Pharm Anal. 2022 Oct;12(5):733-742.
Pruni Semen, the seed of several unique Prunus plants, is a traditional purgative herbal material. To determine the authentic sources of Pruni Semen, 46 samples from four species were collected and analyzed. Ten compounds including Multiflorin A (Mul A), a notable purative compound, were isolated and identified by chemical separation and nuclear magnetic resonance spectroscopy. Seventy-six communal components were identified by ultra-high performance liquid chromatography with linear ion trap-quadrupole Orbitrap mass spectrometry, and acetyl flavonoid glycosides were recognized as characteristic constituents. The flavonoids were distributed in the seed coat and cyanogenic glycosides in the kernel. Based on this, methods for identifying Pruni Semen from different sources were established using chemical fingerprinting, quantitative analysis of the eight principal compounds, hierarchical cluster analysis, principal component analysis, and orthogonal partial least squares discriminant analysis. The results showed that the samples were divided into two categories: one is the small seeds from Prunus humilis (Ph) and Prunus japonica (Pj), and the other is the big seeds from Prunus pedunculata (Pp) and Prunus triloba (Pt). The average content of Mul A was 3.02, 6.93, 0.40, and 0.29 mg/g, while the average content of amygdalin was 18.5, 17.7, 31.5, and 30.9 mg/g in Ph, Pj, Pp, and Pt, respectively. All the above information suggests that small seeds might be superior sources of Pruni Semen. This is the fi rst comprehensive report on the identi fi cation of chemical components in Pruni Semen from different species.
Flavonol glycosides of Rosa multiflora regulates intestinal barrier function through inhibiting claudin expression in differentiated Caco-2 cells.[Pubmed:31767206]
Nutr Res. 2019 Dec;72:92-104.
Eijitsu, the fruits of Rosa multiflora Thunberg, is a traditional Japanese natural medicine and used as purgatives. The active constituents were identified as flavonol glycosides, Multiflorin A (MF), and multinoside A (MSA), but mechanism of the purgative action is still unknown. We hypothesized that the flavonol glycosides 1 and 2 may exhibit the purgative actions through modulating intestinal epithelial barrier function. Then, this study aimed to investigate their effects on intestinal epithelial barrier function and possible molecular mechanisms in human intestinal Caco-2 cells. MF and MSA decreased transepithelial electrical resistance and increased paracellular permeability of Caco-2 cell monolayers. Expression of claudins (CLDNs) involved in paracellular permeability of ions and low-molecular substances was significantly decreased by the treatment with MF or MSA. The compounds increased the ratio of N-cadherin/E-cadherin, expression of transforming growth factor-beta and Slug, and phosphorylation level of Smad3, suggesting epithelial-mesenchymal transition activation, and epithelial-mesenchymal transition inhibition by transforming growth factor-beta receptor kinase inhibitors completely recovered the decreased CLDNs expression caused by MF and MSA. Moreover, the increased paracellular permeability and the decreased CLDNs expression by the treatment with MF or MSA for 24 hours recovered to the same extent as the untreated group with the compounds by continuous culture in the growth medium alone for 48 hours. These results suggest that Eijitsu may be effective in preventing or relieving constipation symptoms, unless used chronically.
Phytochemical characterization of Rosa multiflora Thunb. (Rosaceae) in Japan and South Korea, with a focus on the bioactive flavonol glycoside 'multiflorin A'.[Pubmed:30949951]
J Nat Med. 2019 Jun;73(3):555-565.
Dried achene or anthocarpous accessory fruits of Rosa multiflora Thunb., Rosae fructus ("Eijitsu" in Japanese), have been used in clinical practice to improve constipation within traditional Japanese medicine. Recently, it has been claimed that the efficacy of this crude drug is decreasing, and Multiflorin A, the purgative component, was not detected within the tested samples. In order to clarify the causes of this issue, we investigated Rosa section Synstylae (Rosaceae), including R. multiflora, growing in Japan and South Korea with a focus on the secondary metabolite, Multiflorin A. We recognize that there are two chemotypes based on the presence (Type I) or absence (Type II) of Multiflorin A. Type I contains quercitrin, multinoside A, multiflorin B, and multinoside A acetate as major index compounds. Type II contains hyperin, isoquercitrin, quercetin 3-O-glucuronide, and 3'-methoxy-isoquercitrin as the major index compounds. The chemotype of Rosa section Synstylae (Rosaceae) plants collected in Japan (excluding Tsushima Island) were all classified as Type I with exception of two species, R. luciae and R. sambucina. On the other hand, both Type I and Type II were detected within Rosae fructus obtained from R. multiflora collected in South Korea and Tsushima Island, Japan. The results indicate that Rosae fructus from R. multiflora (Type I) from Japan, excluding Tsushima Island, should be employed clinically, which we describe as purgative.
Peach leaf contains multiflorin a as a potent inhibitor of glucose absorption in the small intestine in mice.[Pubmed:22863923]
Biol Pharm Bull. 2012;35(8):1264-8.
Peach leaf extract has anti-hyperglycemic effects on the postprandial blood glucose level in glucose-loaded mice. In our previous study, the mechanism of action was considered to be the inhibition of glucose absorption in the small intestine. To elucidate the active principle in peach leaf, purification of the active compound and a structure determination were performed. With the use of bioassay-guided fractionation using glucose-loaded mice, the acetylated kaempferol glycoside Multiflorin A (MFA), a potent inhibitor of glucose absorption from the intestine, was isolated from the MeOH extract of leaf of the edible peach Prunus persica. The structure was identified by HPLC using thiazolizine derivatives and by an analysis of its spectral data. The inhibitory effect of MFA against glucose absorption was demonstrated in the dose dependent manner in mice. However, as the deacetylated analog of MFA, multiflorin B did not show the activity at the in vivo, the activity of MFA was suggested to depend on the acetyl group on the sugar moiety. This is the first report of anti-hyperglycemic activity of MFA in peach leaf extract. MFA may be useful in functional foods or medicines for preventing the postprandial absorption of glucose in hyperglycemia.
Isolation and evaluation of kaempferol glycosides from the fern Neocheiropteris palmatopedata.[Pubmed:20100622]
Phytochemistry. 2010 Apr;71(5-6):641-7.
Kaempferol glycosides, named palmatosides A (1), B (2) and C (3), together with three known kaempferol glycosides, multiflorins A (4) and B (5), and afzelin (6), were isolated from the roots of the fern Neocheiropteris palmatopedata. Palmatosides A (1) and B (2) each possessed an unusual sugar moiety containing a 4,4-dimethyl-3-oxo-butoxy substituent group. The isolated compounds were evaluated for their cancer chemopreventive potential based on their ability to inhibit tumor necrosis factor alpha (TNF-alpha)-induced NF-kappaB activity, nitric oxide (NO) production, aromatase, quinone reductase 2 (QR2) and COX-1/-2 activities. Palmatosides B (2) and C (3) inhibited TNF-alpha-induced NF-kappaB activity with IC(50) values of 15.7 and 24.1 microM, respectively; Multiflorin A (4) inhibited aromatase enzyme with an IC(50) value of 15.5 microM; afzelin (6) showed 68.3% inhibition against QR2 at a concentration of 11.5 microg/ml; palmatoside A (1) showed 52% inhibition against COX-1 enzyme at a concentration of 10 microg/ml; and multiflorin B (5) showed 52% inhibition against nitric oxide production at a concentration of 20 microg/ml. In addition, compounds 3-6 were shown to bind QR2 enzyme using LC-MS ultrafiltration binding assay.
Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg.[Pubmed:18449498]
Arch Pharm Res. 2008 Apr;31(4):424-8.
Chemical investigation of the 80% Me(2)CO extract from the seeds of Prunus tomentosa led to the isolation and identification of six flavonoids: kaempferol (1), kaempferol 3-O-alpha-L-rhamnopyranoside (2; afzelin), kaempferol 3-O-beta-D-(6-acetyl)-glucopyranosyl(1-->4)-alpha-L-rhamnopyranoside (3; Multiflorin A), kaempferol 3-O-beta-D-glucopyranosyl(1-->4)-alpha-L-rhamnopyranoside (4; multiflorin B), quercetin 3-O-alpha-L-rhamnopyranoside (5; quercitrin), and quercetin 3-O-beta-D-glucopyranosyl (1-->4)-alpha-L-rhamnopyranoside (6; multinoside A). Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E(2) production in interferon-gamma (INF-gamma) and lipopolysaccharide (LPS)-activated RAW 264.7 cells in vitro (COX-2) of the isolated compounds were evaluated. Compounds 1, 5, and 6 exhibited potent anti-oxidative activity in the DPPH radical scavenging assay with IC(50) values of 57.2, 59.4, and 54.3 microg/mL respectively. The positive control, ascorbic acid, had an IC(50) of 55.5 mug/mL. Compounds 1, 5, and 6 also reduced COX-2 levels in a dose dependent manner with IC(50) values of 10.2, 8.7, and 9.6 microg/mL respectively, with the positive control, indomethacin, having an IC(50) of 5.1 microg/mL. All six compounds inhibited NO production in a dose dependent manner with IC(50) values of 35.1, 42.8, 40.0, 44.8, 43.7, and 43.9 microg/mL respectively, while the positive control, L-NMMA, had an IC(50) of 42.1 microg/mL.


