MethyllycaconitineCAS# 21019-30-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21019-30-7 | SDF | Download SDF |
| PubChem ID | 166177171.0 | Appearance | Powder |
| Formula | C37H50N2O10 | M.Wt | 682.81 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
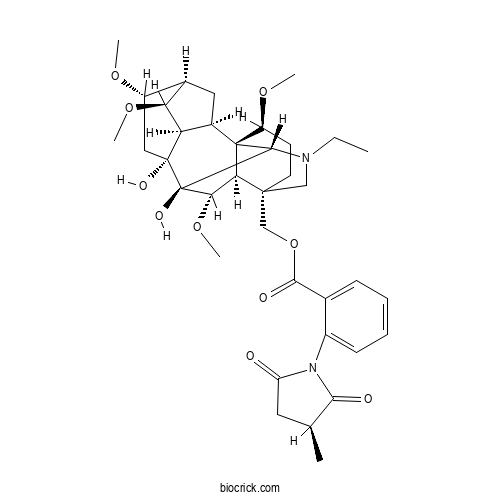
| Chemical Name | [(1R,2R,3R,4S,5R,6S,8R,9S,10S,13S,16S,17R,18S)-11-ethyl-8,9-dihydroxy-4,6,16,18-tetramethoxy-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecan-13-yl]methyl 2-[(3S)-3-methyl-2,5-dioxopyrrolidin-1-yl]benzoate | ||
| SMILES | CCN1CC2(CCC(C34C2C(C(C31)(C5(CC(C6CC4C5C6OC)OC)O)O)OC)OC)COC(=O)C7=CC=CC=C7N8C(=O)CC(C8=O)C | ||
| Standard InChIKey | XLTANAWLDBYGFU-HTWFBASDSA-N | ||
| Standard InChI | InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,19,21-22,24-25,27-30,33,43-44H,7,12-18H2,1-6H3/t19-,21+,22+,24-,25-,27+,28-,29+,30-,33-,34-,35+,36+,37+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyllycaconitine Dilution Calculator

Methyllycaconitine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4645 mL | 7.3227 mL | 14.6454 mL | 29.2907 mL | 36.6134 mL |
| 5 mM | 0.2929 mL | 1.4645 mL | 2.9291 mL | 5.8581 mL | 7.3227 mL |
| 10 mM | 0.1465 mL | 0.7323 mL | 1.4645 mL | 2.9291 mL | 3.6613 mL |
| 50 mM | 0.0293 mL | 0.1465 mL | 0.2929 mL | 0.5858 mL | 0.7323 mL |
| 100 mM | 0.0146 mL | 0.0732 mL | 0.1465 mL | 0.2929 mL | 0.3661 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lycaconitine
Catalog No.:BCX1325
CAS No.:25867-19-0
- Anhydronotoptol
Catalog No.:BCX1324
CAS No.:88206-51-3
- Decuroside V
Catalog No.:BCX1323
CAS No.:96648-59-8
- 5-MethoxyPinocembroside
Catalog No.:BCX1322
CAS No.:1450878-89-3
- Wushanicaritin
Catalog No.:BCX1321
CAS No.:521-45-9
- 3',4',7-Trimethoxyquercetin
Catalog No.:BCX1320
CAS No.:6068-80-0
- Anthranoyllycoctonine
Catalog No.:BCX1319
CAS No.:22413-78-1
- Ethyl ganoderate A
Catalog No.:BCX1318
CAS No.:2242593-18-4
- Ethyl ganoderenate D
Catalog No.:BCX1317
CAS No.:2226858-23-5
- Tunicoidine A
Catalog No.:BCX1316
CAS No.:1415979-39-3
- Rabdosia acid A
Catalog No.:BCX1315
CAS No.:1884697-13-5
- Neoartanin
Catalog No.:BCX1314
CAS No.:104196-69-2
- 6-Methylrhein
Catalog No.:BCX1327
CAS No.:401621-27-0
- Multiflorin B
Catalog No.:BCX1328
CAS No.:52657-01-9
- Multiflorin A
Catalog No.:BCX1329
CAS No.:1350028-90-8
- Quinquenoside III
Catalog No.:BCX1330
CAS No.:208764-53-8
- Pseudoginsenoside RC1
Catalog No.:BCX1331
CAS No.:102805-32-3
- β-D-Glucopyranoside, (3β,12β)-20-[(6-O-α-L-arabinofuranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-, 6-acetate
Catalog No.:BCX1332
CAS No.:1613477-95-4
- Pseudoginsenoside F8
Catalog No.:BCX1333
CAS No.:69884-01-1
- Ajuforrestin B
Catalog No.:BCX1334
CAS No.:708277-48-9
- Ilexoside XLVIII
Catalog No.:BCX1335
CAS No.:129095-76-7
- Gosferol
Catalog No.:BCX1336
CAS No.:37551-62-5
- rel-(+)-(1R,2Z,7Z,10S,11S)-10-(Acetyloxy)-7,12,12-trimethylbicyclo[9.1.0]dodeca-2,7-dien-4-one
Catalog No.:BCX1337
CAS No.:886439-01-6
- Phloyoside I
Catalog No.:BCX1338
CAS No.:139757-58-7
Electroacupuncture ameliorates gastrointestinal dysfunction by modulating DMV cholinergic efferent signals to drive the vagus nerve in p-MCAO rats.[Pubmed:38638995]
Heliyon. 2024 Apr 9;10(8):e29426.
BACKGROUND: The use of proton pump inhibitors in the acute phase of cerebral infarction may lead to adverse long-term outcomes, this study aims to explore the potential of electroacupuncture (EA) in replacing omeprazole in exerting post-stroke gastrointestinal protection. METHODS: A permanent middle cerebral artery infarction model was established using the modified Longa thread occlusion technique. Gastrointestinal motility, gastrointestinal mucosal damage, cerebral infarct volume, and alterations in choline acetyltransferase (ChAT)-positive neurons within the dorsal motor nucleus of the vagus nerve (DMV) were assessed after 7 days of EA at Zusanli (ST36) or omeprazole intervention. To evaluate the role of the vagal nerve in mitigating post-stroke gastrointestinal dysfunction, we employed subdiaphragmatic vagotomy and the ChAT-specific inhibitor alpha-NETA. Additionally, we utilized Methyllycaconitine (MLA), a selective inhibitor of the alpha7-type nicotinic acetylcholine receptor (alpha7nAChR), and PNU282987, an agonist, to identify the target of EA. RESULTS: EA restored ChAT neurons lost in the DMV, activated the vagus nerve and conferred cerebroprotection while ameliorating gastrointestinal mucosal injury and gastrointestinal motility disorders. In addition, following the administration of the alpha7nAChR antagonist, the attenuation of gastric mucosal injury and inflammatory factors induced by EA was hindered, although gastrointestinal motility still exhibited improvement. CONCLUSION: EA at ST36 promotes the restoration of cholinergic signaling in the DMV of stroke-afflicted rats, and its excitation of the vagal nerve inhibits gastrointestinal inflammation after stroke via alpha7nAChR, while improvement in gastrointestinal motility could be mediated by other acetylcholine receptors.
Activation of alpha-7 nicotinic acetylcholine receptor by tropisetron mitigates 3-nitropropionic acid-induced Huntington's disease in rats: Role of PI3K/Akt and JAK2/NF-kappaB signaling pathways.[Pubmed:38513929]
Chem Biol Interact. 2024 Apr 25;393:110957.
Huntington's disease (HD) is an inheritable autosomal-dominant disorder that targets mainly the striatum. 3-Nitropropionic acid (3-NP) induces obvious deleterious behavioral, neurochemical, and histological effects similar to the symptoms of HD. Our study aimed to examine the neuroprotective activity of tropisetron, an alpha-7 neuronal nicotinic acetylcholine receptor (alpha-7nAChR) agonist, against neurotoxic events associated with 3-NP-induced HD in rats. Forty-eight rats were randomly allocated into four groups. Group I received normal saline, while Groups II, III and IV received 3-NP for 2 weeks. In addition, Group III and IV were treated with tropisetron 1 h after 3-NP administration. Meanwhile, Group IV received Methyllycaconitine (MLA), an alpha-7nAChR antagonist, 30 min before tropisetron administration. Treatment with tropisetron improved motor deficits as confirmed by the behavioral tests and restored normal histopathological features of the striatum. Moreover, tropisetron showed an anti-oxidant activity via increasing the activities of SDH and HO-1 as well as Nrf2 expression along with reducing MDA level. Tropisetron also markedly upregulated the protein expression of p-PI3K and p-Akt which in turn hampered JAK2/NF-kappaB inflammatory cascade. In addition, tropisetron showed an anti-apoptotic activity through boosting the expression of Bcl-2 and reducing Bax expression and caspase-3 level. Interestingly, all the aforementioned effects of tropisetron were blocked by pre-administration of MLA, which confirms that such neuroprotective effects are mediated via activating of alpha-7nAChR. In conclusion, tropisetron showed a neuroprotective activity against 3-NP-induced HD via activating PI3K/Akt signaling and suppressing JAK2/NF-kappaB inflammatory axis. Thus, repositioning of tropisetron could represent a promising therapeutic strategy in management of HD.
Vagus nerve stimulation as a promising neuroprotection for ischemic stroke via alpha7nAchR-dependent inactivation of microglial NLRP3 inflammasome.[Pubmed:38504011]
Acta Pharmacol Sin. 2024 Mar 19.
Ischemic stroke is a major cause of disability and death worldwide, and its management requires urgent attention. Previous studies have shown that vagus nerve stimulation (VNS) exerts neuroprotection in ischemic stroke by inhibiting neuroinflammation and apoptosis. In this study, we evaluated the timing for VNS intervention in ischemic stroke, and the underlying mechanisms of VNS-induced neuroprotection. Mice were subjected to transient middle cerebral artery occlusion (tMCAO) for 60 min. The left vagus nerve at cervical level was exposed and attached to an electrode connected to a low-frequency electrical stimulator. Vagus nerve stimulation (VNS) was given for 60 min before, during and after tMCAO (Pre-VNS, Dur-VNS, Post-VNS). Neurological function was assessed 24 h after reperfusion. We found that all the three VNS significantly protected against the tMCAO-induced injury evidenced by improved neurological function and reduced infarct volume. Moreover, the Pre-VNS was the most effective against the ischemic injury. We found that tMCAO activated microglia in the ischemic core and penumbra regions of the brain, followed by the NLRP3 inflammasome activation-induced neuroinflammation, which finally triggered neuronal death. VNS treatment preserved alpha7nAChR expression in the penumbra regions, inhibited NLRP3 inflammasome activation and ensuing neuroinflammation, rescuing cerebral neurons. The role of alpha7nAChR in microglial NLRP3 inflammasome activation in ischemic stroke was further validated using genetic manipulations, including Chrna7 knockout mice and microglial Chrna7 overexpression mice, as well as pharmacological interventions using the alpha7nAChR inhibitor Methyllycaconitine and agonist PNU-282987. Collectively, this study demonstrates the potential of VNS as a safe and effective strategy to treat ischemic stroke, and presents a new approach targeting microglial NLRP3 inflammasome, which might be therapeutic for other inflammation-related diseases.
Noninvasive neuromodulation protects against doxorubicin-induced cardiotoxicity and inhibits tumor growth.[Pubmed:38425841]
iScience. 2024 Feb 13;27(3):109163.
Doxorubicin (Dox) poses a considerable threat to patients owing to its cardiotoxicity, thus limiting its clinical utility. Optimal cardioprotective intervention strategies are needed to suppress tumor growth but also minimize cardiac side effects. Here, we showed that tragus vagus nerve stimulation (tVNS) improved the imbalanced autonomic tone, ameliorated impaired cardiac function and fibrosis, attenuated myocyte apoptosis, and mitochondrial dysfunction compared to those in the Dox group. The beneficial effects were attenuated by Methyllycaconitine citrate (MLA). The transcript profile revealed that there were 312 differentially expressed genes and the protection of tVNS and retardation of MLA were related to inflammatory response and NADPH oxidase activity. In addition, tVNS synergizing with Dox inhibited tumor growth and lung metastasis and promoted apoptosis of tumor cells in an anti-tumor immunity manner. These results indicated that non-invasive neuromodulation can play a dual role in preventing Dox-induced cardiotoxicity and suppressing tumor growth through inflammation and oxidative stress.
Critical roles of nicotinic acetylcholine receptors in olfactory memory formation and retrieval in crickets.[Pubmed:38405118]
Front Physiol. 2024 Feb 9;15:1345397.
Acetylcholine (ACh) is a major excitatory neurotransmitter in the insect central nervous system, and insect neurons express several types of ACh receptors (AChRs). AChRs are classified into two subgroups, muscarinic AChRs and nicotinic AChRs (nAChRs). nAChRs are also divided into two subgroups by sensitivity to alpha-bungarotoxin (alpha-BGT). The cricket Gryllus bimaculatus is one of the useful insects for studying the molecular mechanisms in olfactory learning and memory. However, the roles of nAChRs in olfactory learning and memory of the cricket are still unknown. In the present study, to investigate whether nAChRs are involved in cricket olfactory learning and memory, we tested the effects of two different AChR antagonists on long-term memory (LTM) formation and retrieval in a behavioral assay. The two AChR antagonists that we used are mecamylamine (MEC), an alpha-BGT-insensitive nAChR antagonist, and Methyllycaconitine (MLA), an alpha-BGT-sensitive nAChR antagonist. In crickets, multiple-trial olfactory conditioning induced 1-day memory (LTM), whereas single-trial olfactory conditioning induced 1-h memory (mid-term memory, MTM) but not 1-day memory. Crickets injected with MEC 20 min before the retention test at 1 day after the multiple-trial conditioning exhibited no memory retrieval. This indicates that alpha-BGT-insensitive nAChRs participate in memory retrieval. In addition, crickets injected with MLA before the multiple-trial conditioning exhibited MTM but not LTM, indicating that alpha-BGT-sensitive nAChRs participate in the formation of LTM. Moreover, injection of nicotine (an nAChR agonist) before the single-trial conditioning induced LTM. Finally, the nitric oxide (NO)-cGMP signaling pathway is known to participate in the formation of LTM in crickets, and we conducted co-injection experiments with an agonist or inhibitor of the nAChR and an activator or inhibitor of the NO-cGMP signaling pathway. The results suggest that nAChR works upstream of the NO-cGMP signaling system in the LTM formation process.
Combined administration of anisodamine and neostigmine alleviated colitis by inducing autophagy and inhibiting inflammation.[Pubmed:38354108]
PLoS One. 2024 Feb 14;19(2):e0291543.
Our previous work demonstrated that the anisodamine (ANI) and neostigmine (NEO) combination produced an antiseptic shock effect and rescued acute lethal crush syndrome by activating the alpha7 nicotinic acetylcholine receptor (alpha7nAChR). This study documents the therapeutic effect and underlying mechanisms of the ANI/NEO combination in dextran sulfate sodium (DSS)-induced colitis. Treating mice with ANI and NEO at a ratio of 500:1 alleviated the DSS-induced colitis symptoms, reduced body weight loss, improved the disease activity index, enhanced colon length, and alleviated colon inflammation. The combination treatment also enhanced autophagy in the colon of mice with DSS-induced colitis and lipopolysaccharide/DSS-stimulated Caco-2 cells. Besides, the ANI/NEO treatment significantly reduced INF-gamma, TNF-alpha, IL-6, and IL-22 expression in colon tissues and decreased TNF-alpha, IL-1beta, and IL-6 mRNA levels in Caco-2 cells. Meanwhile, the autophagy inhibitor 3-methyladenine and ATG5 siRNA attenuated these effects. Furthermore, 3-methyladenine (3-MA) and the alpha7nAChR antagonist Methyllycaconitine (MLA) weakened the ANI/NEO-induced protection on DSS-induced colitis in mice. Overall, these results indicate that the ANI/NEO combination exerts therapeutic effects through autophagy and alpha7nAChR in a DSS-induced colitis mouse model.
(18)F-ASEM PET/MRI targeting alpha7-nicotinic acetylcholine receptor can reveal skeletal muscle denervation.[Pubmed:38252356]
EJNMMI Res. 2024 Jan 22;14(1):8.
BACKGROUND: The increased expression of the nicotinic acetylcholine receptor (nAChR) in muscle denervation is thought to be associated with electrophysiological acetylcholine supersensitivity after nerve injury. Hence, we investigated the utility of the (18)F-ASEM alpha7-nAChR targeting radiotracer as a new diagnostic method by visualizing skeletal muscle denervation in mouse models of sciatic nerve injury. METHODS: Ten-week-old C57BL/6 male mice were utilized. The mice were anesthetized, and the left sciatic nerve was resected after splitting the gluteal muscle. One week (n = 11) and three weeks (n = 6) after the denervation, (18)F-ASEM positron emission tomography/magnetic resonance imaging (PET/MRI) was acquired. Maximum standardized uptake values (SUVmax) of the tibialis anterior muscle were measured for the denervated side and the control side. Autoradiographic evaluation was performed to measure the mean counts of the denervated and control tibialis anterior muscles at one week. In addition, immunohistochemistry was used to identify alpha7-nAChR-positive areas in denervated and control tibialis anterior muscles at one week (n = 6). Furthermore, a blocking study was conducted with Methyllycaconitine (MLA, n = 5). RESULTS: (18)F-ASEM PET/MRI showed significantly increased (18)F-ASEM uptake in the denervated tibialis anterior muscle relative to the control side one week and three weeks post-denervation. SUVmax of the denervated muscles at one week and three weeks showed significantly higher uptake than the control (P = 0.0033 and 0.0277, respectively). The relative uptake by autoradiography for the denervated muscle was significantly higher than in the control, and immunohistochemistry revealed significantly greater alpha7-nAChR expression in the denervated muscle (P = 0.0277). In addition, the blocking study showed no significant (18)F-ASEM uptake in the denervated side when compared to the control (P = 0.0796). CONCLUSIONS: Our results suggest that nAChR imaging with (18)F-ASEM has potential as a noninvasive diagnostic method for peripheral nervous system disorders.
Galantamine ameliorates experimental pancreatitis.[Pubmed:37907853]
Mol Med. 2023 Oct 31;29(1):149.
BACKGROUND: Acute pancreatitis is a common and serious inflammatory condition currently lacking disease modifying therapy. The cholinergic anti-inflammatory pathway (CAP) is a potent protective anti-inflammatory response activated by vagus nerve-dependent alpha7 nicotinic acetylcholine receptor (alpha7nAChR) signaling using splenic CD4(+) T cells as an intermediate. Activating the CAP ameliorates experimental acute pancreatitis. Galantamine is an acetylcholinesterase inhibitor (AChEI) which amplifies the CAP via modulation of central muscarinic ACh receptors (mAChRs). However, as mAChRs also activate pancreatitis, it is currently unknown whether galantamine would be beneficial in acute pancreatitis. METHODS: The effect of galantamine (1-6 mg/kg-body weight) on caerulein-induced acute pancreatitis was evaluated in mice. Two hours following 6 hourly doses of caerulein (50 microg/kg-body weight), organ and serum analyses were performed with accompanying pancreatic histology. Experiments utilizing vagotomy, gene knock out (KO) technology and the use of nAChR antagonists were also performed. RESULTS: Galantamine attenuated pancreatic histologic injury which was mirrored by a reduction in serum amylase and pancreatic inflammatory cytokines and an increase the anti-inflammatory cytokine IL-10 in the serum. These beneficial effects were not altered by bilateral subdiaphragmatic vagotomy, KO of either choline acetyltransferase(+) T cells or alpha7nAChR, or administration of the nAChR ganglionic blocker mecamylamine or the more selective alpha7nAChR antagonist Methyllycaconitine. CONCLUSION: Galantamine improves acute pancreatitis via a mechanism which does not involve previously established physiological and molecular components of the CAP. As galantamine is an approved drug in widespread clinical use with an excellent safety record, our findings are of interest for further evaluating the potential benefits of this drug in patients with acute pancreatitis.
Anti-smoking drugs cytisine and varenicline reduce cardiac reperfusion injury in rat model of myocardial ischemia.[Pubmed:37871826]
Biochimie. 2024 Jan;216:108-119.
Evidence to date indicates that activation of nicotinic acetylcholine receptors (nAChRs) can reduce cardiac injury from ischemia and subsequent reperfusion. The use of nAChR agonists in various animal models leads to a reduction in reperfusion injury. Earlier this effect was shown for the agonists of alpha7 nAChR subtype. In this work, we demonstrated the expression of mRNA encoding alpha4, alpha6 and beta2 nAChR subunits in the left ventricle of rat heart. In a rat model of myocardial ischemia, we studied the effect of alpha4beta2 nAChR agonists cytisine and varenicline, medicines used for the treatment of nicotine addiction, and found them to significantly reduce myocardium ischemia-reperfusion injury, varenicline manifesting a higher protection. Dihydro-beta-erythroidine, antagonist of alpha4beta2 nAChR, as well as Methyllycaconitine, antagonist of alpha7 and alpha6beta2-containing nAChR, prevented protective effect of varenicline. This together with the presence of alpha4, alpha6 and beta2 subunit mRNA in the left ventricule of rat heart raises the possibility that the varenicline effect is mediated by alpha4beta2 as well as by alpha7 and/or alpha6beta2-containing receptors. Our results point to a new way for the use of cytisine and varenicline as cardioprotective agents.
Vagus Nerve Stimulation Relives Irritable Bowel Syndrome and the Associated Depression via alpha7nAChR-mediated Anti-inflammatory Pathway.[Pubmed:37625687]
Neuroscience. 2023 Oct 15;530:26-37.
OBJECTIVES: The present study is designed to investigate the role of vagus nerve in the treatments of irritable bowel syndrome (IBS) and the associated central nervous system disorders. METHODS: An IBS animal model was established by giving acetic acid and chronic-acute stress (AA-CAS) treatment in adult male Wistar rats. Subdiaphragmatic vagotomy (SDV) and vagus nerve stimulation (VNS) were performed to intervene the excitability of vagus nerve. Permeability of blood brain barrier (BBB) was measured and agonist and antagonist of alpha7 nicotinic acetylcholine receptor (alpha7nAChR) were used to explore the relevant mechanisms. RESULTS: AA-CAS treatment resulted in abnormal fecal output, increased visceral sensitivity, depressive-like behaviors, and overexpression of inflammatory mediators, all of which were reversed by VNS treatment. The effects of VNS could also be observed when alpha7nAChR agonist was applied. Whereas alpha7nAChR antagonist (Methyllycaconitine, MLA) reversed VNS's effects. Interestingly, VNS also reduced the increased permeability of blood brain barrier (BBB) following AA-CAS treatment in IBS rats. SDV treatment only show temporary efficacy on AA-CAS-induced symptoms and had no effect on the permeability of BBB. CONCLUSION: The intestinal abnormalities and depressive symptoms in IBS rats can be improved by VNS treatment. This positive effect of VNS was achieved through alpha7nAChR-mediated inflammatory pathway and may also be associated with the decreased of BBB permeability.
Nicotine Decreases Nerve Regeneration and Pain Behaviors via PTEN and Downstream Inflammation-Related Pathway in Two Rat Nerve Injury Models.[Pubmed:37620149]
eNeuro. 2023 Sep 6;10(9):ENEURO.0185-23.2023.
Neuropathic pain is stubborn and associated with the peripheral nerve regeneration process. Nicotine has been found to reduce pain, but whether it is involved in the regulation of nerve regeneration and the underlying mechanism are unknown. In this study, we examined the mechanical allodynia thermal hyperalgesia together with the peripheral nerve regeneration after nicotine exposure in two rat neuropathic pain models. In the spinal nerve ligation model, in which anatomic nerve regeneration can be easily observed, nicotine reduced anatomic measures of regeneration as well as expression of regeneration marker growth-associated protein 43 (GAP43). In the tibial nerve crush model, nicotine treatment significantly suppressed GAP43 expression and functional reinnervation as measured by myelinated action potential and electromyography of gastrocnemius. In both models, nicotine treatment reduced macrophage density in the sensory ganglia and peripheral nerve. These effects of nicotine were reversed by the selective alpha7 nicotinic acetylcholine receptor (nAChR) blocker Methyllycaconitine. In addition, nicotine significantly elevated expression of PTEN (the phosphatase and tensin homolog deleted on chromosome 10), a key player in both regeneration and pain. Pharmacological interference of PTEN could regulate GAP43 expression, pain-related behaviors, and macrophage infiltration in a nicotine-treated nerve crush model. Our results reveal that nicotine and its alpha7-nAChR regulate both peripheral nerve regeneration process and pain though PTEN and the downstream inflammation-related pathway.
(R,S)-trihexyphenidyl, acting via a muscarinic receptor-independent mechanism, inhibits hippocampal glutamatergic and GABAergic synaptic transmissions: Potential relevance for treatment of organophosphorus intoxication.[Pubmed:37549771]
Neuropharmacology. 2023 Nov 15;239:109684.
Preclinical studies have reported that, compared to the muscarinic receptor (mAChR) antagonist atropine, (R,S)-trihexyphenidyl (THP) more effectively counters the cholinergic crisis, seizures, and neuropathology triggered by organophosphorus (OP)-induced acetylcholinesterase (AChE) inhibition. The greater effectiveness of THP was attributed to its ability to block mAChRs and N-methyl-d-aspartate-type glutamatergic receptors (NMDARs) in the brain. However, THP also inhibits alpha7 nicotinic receptors (nAChRs). The present study examined whether THP-induced inhibition of mAChRs, alpha7 nAChRs, and NMDARs is required to suppress glutamatergic synaptic transmission, whose overstimulation sustains OP-induced seizures. In primary hippocampal cultures, THP (1-30 muM) suppressed the frequency of excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs, respectively) recorded from neurons in nominally Mg(2+)-free solution. A single sigmoidal function adequately fit the overlapping concentration-response relationships for THP-induced suppression of IPSC and EPSC frequencies yielding an IC50 of 6.3 +/- 1.3 muM. Atropine (1 muM), the NMDAR antagonist d,l-2-amino-5-phosphonopentanoic acid (D,L-AP5, 50 muM), and the alpha7 nAChR antagonist Methyllycaconitine (MLA, 10 nM) did not prevent THP-induced inhibition of synaptic transmission. THP (10 muM) did not affect the probability of transmitter release because it had no effect on the frequency of miniature IPSCs and EPSCs recorded in the presence of tetrodotoxin. Additionally, THP had no effect on the amplitudes and decay-time constants of miniature IPSCs and EPSCs; therefore, it did not affect the activity of postsynaptic GABA(A) and glutamate receptors. This study provides the first demonstration that THP can suppress action potential-dependent synaptic transmission via a mechanism independent of NMDAR, mAChR, and alpha7 nAChR inhibition.
Nicotine promotes renal interstitial fibrosis via upregulation of XIAP in an alpha7-nAChR-dependent manner.[Pubmed:37451424]
Mol Cell Endocrinol. 2023 Oct 1;576:111989.
Renal fibrosis, characterized by excessive accumulation of the extracellular matrix in the renal tubulointerstitium, can lead to chronic kidney disease (CKD), resulting in a heavy burden on families and society. Clinical studies have shown that smoking is closely associated with CKD deterioration in patients with diabetes, hypertension, polycystic kidney disease, and kidney transplantation. However, the mechanism of action of nicotine in renal fibrosis pathogenesis remains largely unknown. X-linked inhibitor of apoptosis (XIAP), a member of the inhibitor of apoptosis protein (IAP) family, is involved in apoptosis, necroptosis, autophagy, and immune response. Here, the upregulated expression of XIAP and alpha7 nicotine acetylcholine receptor (alpha7-nAChR) was determined in the kidneys of the CKD smoking group in human and animal studies. A significant positive correlation between XIAP and cotinine was observed. In addition, the nuclear translocation and transcriptional activity of SP1 were promoted when nicotine bound to alpha7-nAChR, resulting in XIAP overexpression and renal interstitial fibrosis progression. This phenotype can be reversed by the nicotine receptor subtype alpha7-nAChR antagonists Methyllycaconitine. Our results revealed the complex underlying mechanism of nicotine in promoting renal fibrosis by altering SP1 nucleocytoplasmic translocation and regulating XIAP expression. These results provide novel insights into the pathogenesis and treatment of CKD.


