WushanicaritinCAS# 521-45-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 521-45-9 | SDF | Download SDF |
| PubChem ID | 12310757.0 | Appearance | Powder |
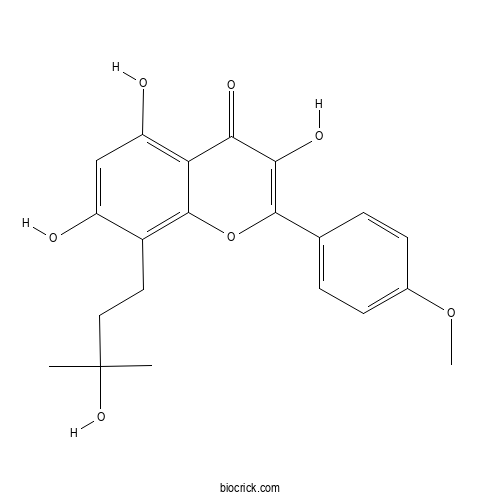
| Formula | C21H22O7 | M.Wt | 386.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5,7-trihydroxy-8-(3-hydroxy-3-methylbutyl)-2-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | CC(C)(CCC1=C2C(=C(C=C1O)O)C(=O)C(=C(O2)C3=CC=C(C=C3)OC)O)O | ||
| Standard InChIKey | VAYWXTLNNGACLF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H22O7/c1-21(2,26)9-8-13-14(22)10-15(23)16-17(24)18(25)19(28-20(13)16)11-4-6-12(27-3)7-5-11/h4-7,10,22-23,25-26H,8-9H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Wushanicaritin Dilution Calculator

Wushanicaritin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.588 mL | 12.94 mL | 25.8799 mL | 51.7598 mL | 64.6998 mL |
| 5 mM | 0.5176 mL | 2.588 mL | 5.176 mL | 10.352 mL | 12.94 mL |
| 10 mM | 0.2588 mL | 1.294 mL | 2.588 mL | 5.176 mL | 6.47 mL |
| 50 mM | 0.0518 mL | 0.2588 mL | 0.5176 mL | 1.0352 mL | 1.294 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2588 mL | 0.5176 mL | 0.647 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3',4',7-Trimethoxyquercetin
Catalog No.:BCX1320
CAS No.:6068-80-0
- Anthranoyllycoctonine
Catalog No.:BCX1319
CAS No.:22413-78-1
- Ethyl ganoderate A
Catalog No.:BCX1318
CAS No.:2242593-18-4
- Ethyl ganoderenate D
Catalog No.:BCX1317
CAS No.:2226858-23-5
- Tunicoidine A
Catalog No.:BCX1316
CAS No.:1415979-39-3
- Rabdosia acid A
Catalog No.:BCX1315
CAS No.:1884697-13-5
- Neoartanin
Catalog No.:BCX1314
CAS No.:104196-69-2
- Poricoic acid BM
Catalog No.:BCX1313
CAS No.:1815623-74-5
- Isooxypeucedanin
Catalog No.:BCX1312
CAS No.:5058-15-1
- Oxypeucedanin hydrate-3”-ethyl ether
Catalog No.:BCX1311
CAS No.:55481-87-3
- Theasaponin E2
Catalog No.:BCX1310
CAS No.:220114-30-7
- 3'-O-Angeloylhamaudol
Catalog No.:BCX1309
CAS No.:84272-84-4
- 5-MethoxyPinocembroside
Catalog No.:BCX1322
CAS No.:1450878-89-3
- Decuroside V
Catalog No.:BCX1323
CAS No.:96648-59-8
- Anhydronotoptol
Catalog No.:BCX1324
CAS No.:88206-51-3
- Lycaconitine
Catalog No.:BCX1325
CAS No.:25867-19-0
- Methyllycaconitine
Catalog No.:BCX1326
CAS No.:21019-30-7
- 6-Methylrhein
Catalog No.:BCX1327
CAS No.:401621-27-0
- Multiflorin B
Catalog No.:BCX1328
CAS No.:52657-01-9
- Multiflorin A
Catalog No.:BCX1329
CAS No.:1350028-90-8
- Quinquenoside III
Catalog No.:BCX1330
CAS No.:208764-53-8
- Pseudoginsenoside RC1
Catalog No.:BCX1331
CAS No.:102805-32-3
- β-D-Glucopyranoside, (3β,12β)-20-[(6-O-α-L-arabinofuranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-, 6-acetate
Catalog No.:BCX1332
CAS No.:1613477-95-4
- Pseudoginsenoside F8
Catalog No.:BCX1333
CAS No.:69884-01-1
Chemical inhibition and stable knock-down of efflux transporters leads to reduced glucuronidation of wushanicaritin in UGT1A1-overexpressing HeLa cells: the role of breast cancer resistance protein (BCRP) and multidrug resistance-associated proteins (MRPs) in the excretion of glucuronides.[Pubmed:29318243]
Food Funct. 2018 Mar 1;9(3):1410-1423.
Active efflux transport of glucuronides out of cells is a critical process in elimination of drugs and food-derived compounds. Wushanicaritin, a natural polyphenol from Epimedium species, has shown many biological activities. However, the transporters responsible for excretion of Wushanicaritin glucuronides still remain undefined. Herein, chemical inhibitors (Ko143, MK571, dipyridamole and leukotriene C4) and single stable knocked-down efflux transporters (BCRP, MRP1, MRP3 and MRP4) were used to determine the contributions of efflux transporters to glucuronide efflux and cellular glucuronidation in UGT1A1-overexpressing HeLa cells (HeLa1A1). Knock-down of transporters was performed by stable transfection of short hairpin RNA (shRNA) using lentiviral vectors. The HeLa1A1 cell lysate catalyzed Wushanicaritin glucuronidation, generating Wushanicaritin-3-O-glucuronide and Wushanicaritin-7-O-glucuronide. Ko143 (a dual inhibitor of BCRP, 5-20 muM) caused a marked decrease in excretion rate (maximal 53.4%) and increase of intracellular glucuronides (maximal 86.0%), while MK-571 (an inhibitor of MRPs, 5-20 muM) resulted in a significant reduction in excretion rate (maximal 64.6%) and rise of intracellular glucuronides (maximal 98.0%). By contrast, dipyridamole and leukotriene C4 showed no inhibitory effects on glucuronide excretion. Furthermore, shRNA-mediated silencing of a target transporter led to a marked reduction in the excretion rate of Wushanicaritin glucuronides (maximal 33.8% for BCRP; 25.9% for MRP1; 26.7% for MRP3; 39.3% for MRP4). Transporter silencing also led to substantial decreases in efflux clearance (maximal 61.5% for BCRP; 48.7% for MRP1; 35.1% for MRP3; 63.1% for MRP4). In conclusion, chemical inhibition and gene silencing results suggested that BCRP, MRP1, MRP3 and MRP4 were significant contributors to excretion of Wushanicaritin glucuronides.
Synthesis of prenylated flavonols and their potents as estrogen receptor modulator.[Pubmed:28963488]
Sci Rep. 2017 Sep 29;7(1):12445.
Prenylated flavonols are known as phytoestrogen and have good bioactivties. However, their abundances in nature are pretty low. It is required to find an efficient synthesis technique. Icariin is a prenylated flavonol glycoside with low cost. It can be used to synthesize different prenylated flavonols. A combination of cellulase and trifluoacetic acid hydrolysis could effectively remove rhamnose and glucose from icariin. Icaritin, anhydroicaritin and Wushanicaritin were the leading prenylated flavonol products. Their affinities to estrogen receptors alpha and beta were predicted by docking study. The weak affinity of Wushanicaritin indicated that prenyl hydroxylation impaired its affinity to estrogen receptor beta. The prenyl cyclization led to a loss of affinity to both receptors. The interactions between icaritin and ligand binding cavity of estrogen receptor beta were simulated. pi-pi stacking and hydrophobic forces were predicted to be the dominant interactions positioning icaritin, which induced the helix (H12) forming an activated conformation.
In Vitro Glucuronidation of Wushanicaritin by Liver Microsomes, Intestine Microsomes and Expressed Human UDP-Glucuronosyltransferase Enzymes.[Pubmed:28925930]
Int J Mol Sci. 2017 Sep 19;18(9):1983.
Wushanicaritin, a natural polyphenol compound, exerts many biological activities. This study aimed to characterize Wushanicaritin glucuronidation by pooled human liver microsomes (HLM), human intestine microsomes and individual uridine diphosphate-glucuronosyltransferase (UGT) enzyme. Glucuronidation rates were determined by incubating Wushanicaritin with uridine diphosphoglucuronic acid-supplemented microsomes. Kinetic parameters were derived by appropriate model fitting. Reaction phenotyping, the relative activity factor (RAF) and activity correlation analysis were performed to identify the main UGT isoforms. Wushanicaritin glucuronidation in HLM was efficient with a high CL(int) (intrinsic clearance) value of 1.25 and 0.69 mL/min/mg for G1 and G2, respectively. UGT1A1 and 1A7 showed the highest activities with the intrinsic clearance (CL(int)) values of 1.16 and 0.38 mL/min/mg for G1 and G2, respectively. In addition, G1 was significantly correlated with beta-estradiol glucuronidation (r = 0.847; p = 0.0005), while G2 was also correlated with chenodeoxycholic acid glucuronidation (r = 0.638, p = 0.026) in a bank of individual HLMs (n = 12). Based on the RAF approach, UGT1A1 contributed 51.2% for G1, and UGT1A3 contributed 26.0% for G2 in HLM. Moreover, glucuronidation of Wushanicaritin by liver microsomes showed marked species difference. Taken together, UGT1A1, 1A3, 1A7, 1A8, 1A9 and 2B7 were identified as the main UGT contributors responsible for Wushanicaritin glucuronidation.
Antioxidant flavonoids from Epimedium wushanense.[Pubmed:21968061]
Fitoterapia. 2012 Jan;83(1):44-8.
Two new flavonoids, Wushanicaritin (1) and wushankaempferol (2), along with 24 known flavonoids were isolated from the whole herb of Epimedium wushanense T.S. Ying (Berberidaceae). On the basis of NMR and ESI-MS spectroscopic analysis, structures of compounds 1 and 2 were elucidated as 8-gamma-hydroxy-gamma,gamma-dimethylpropyl-3,5,7-trihydroxy-4'- methoxyflavone and kaempferol 3-O-alpha-l-[2,3-di-O-beta-D-(6-E-p-coumaroyl) glucopyranosyl]-rhamnopyranosyl-7-O-alpha-L-rhamnopyranoside, respectively. DPPH radical scavenging activity tests indicated that 1 (IC(50) 35.3 muM) exhibited antioxidant activity comparable to Vitamin C (IC(50) 32.0 muM), while 2 (IC(50) 443.7 muM) showed weak activity.


