3-Amino-4-hydroxybenzoic acidCAS# 1571-72-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1571-72-8 | SDF | Download SDF |
| PubChem ID | 65083 | Appearance | Powder |
| Formula | C7H7NO3 | M.Wt | 153 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-amino-4-hydroxybenzoic acid | ||
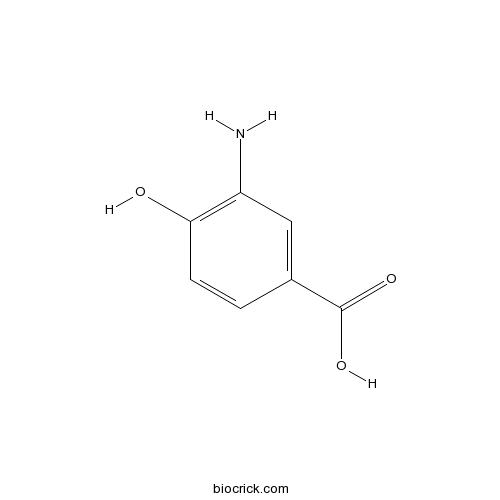
| SMILES | C1=CC(=C(C=C1C(=O)O)N)O | ||
| Standard InChIKey | MRBKRZAPGUCWOS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H7NO3/c8-5-3-4(7(10)11)1-2-6(5)9/h1-3,9H,8H2,(H,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-Amino-4-hydroxybenzoic acid Dilution Calculator

3-Amino-4-hydroxybenzoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.5359 mL | 32.6797 mL | 65.3595 mL | 130.719 mL | 163.3987 mL |
| 5 mM | 1.3072 mL | 6.5359 mL | 13.0719 mL | 26.1438 mL | 32.6797 mL |
| 10 mM | 0.6536 mL | 3.268 mL | 6.5359 mL | 13.0719 mL | 16.3399 mL |
| 50 mM | 0.1307 mL | 0.6536 mL | 1.3072 mL | 2.6144 mL | 3.268 mL |
| 100 mM | 0.0654 mL | 0.3268 mL | 0.6536 mL | 1.3072 mL | 1.634 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lipedoside B-III
Catalog No.:BCC8201
CAS No.:157085-48-8
- SNAP 5089
Catalog No.:BCC7350
CAS No.:157066-77-8
- U 89843A
Catalog No.:BCC7466
CAS No.:157013-85-9
- 5,7-Dihydroxy-3,4',8-trimethoxyflavone
Catalog No.:BCN1551
CAS No.:1570-09-8
- H-D-Arg-OH
Catalog No.:BCC2868
CAS No.:157-06-2
- Wedeliatrilolactone A
Catalog No.:BCN6732
CAS No.:156993-29-2
- Grosvenorin
Catalog No.:BCN1262
CAS No.:156980-60-8
- Isosalicifolin
Catalog No.:BCN6502
CAS No.:156974-99-1
- OSU 6162 hydrochloride
Catalog No.:BCC7424
CAS No.:156907-84-5
- Licofelone
Catalog No.:BCC4432
CAS No.:156897-06-2
- N1,N11-Diethylnorspermine tetrahydrochloride
Catalog No.:BCC6654
CAS No.:156886-85-0
- Ibuprofen
Catalog No.:BCC3791
CAS No.:15687-27-1
- Ajuforrestin A
Catalog No.:BCN8008
CAS No.:157110-18-4
- Noopept
Catalog No.:BCC1804
CAS No.:157115-85-0
- Harzianic acid
Catalog No.:BCN1838
CAS No.:157148-06-6
- 3-Amino-5-phenylpyrazole
Catalog No.:BCC8616
CAS No.:1572-10-7
- Bosentan Hydrate
Catalog No.:BCC4494
CAS No.:157212-55-0
- ML-323
Catalog No.:BCC4313
CAS No.:1572414-83-5
- Travoprost
Catalog No.:BCC5189
CAS No.:157283-68-6
- DMP 777
Catalog No.:BCC1534
CAS No.:157341-41-8
- MAP4
Catalog No.:BCC6758
CAS No.:157381-42-5
- Bacoside A3
Catalog No.:BCC8128
CAS No.:157408-08-7
- Taxuspine B
Catalog No.:BCN6938
CAS No.:157414-05-6
- Coronarin D methyl ether
Catalog No.:BCN1705
CAS No.:157528-81-9
3-Amino-4-hydroxybenzoic acid production from sweet sorghum juice by recombinant Corynebacterium glutamicum.[Pubmed:26409852]
Bioresour Technol. 2015 Dec;198:410-7.
The production of the bioplastic precursor 3-Amino-4-hydroxybenzoic acid (3,4-AHBA) from sweet sorghum juice, which contains amino acids and the fermentable sugars sucrose, glucose and fructose, was assessed to address the limitations of producing bio-based chemicals from renewable feedstocks. Recombinant Corynebacterium glutamicum strain KT01 expressing griH and griI derived from Streptomyces griseus produced 3,4-AHBA from the sweet sorghum juice of cultivar SIL-05 at a final concentration (1.0 g l(-1)) that was 5-fold higher than that from pure sucrose. Fractionation of sweet sorghum juice by nanofiltration (NF) membrane separation (molecular weight cut-off 150) revealed that the NF-concentrated fraction, which contained the highest concentrations of amino acids, increased 3,4-AHBA production, whereas the NF-filtrated fraction inhibited 3,4-AHBA biosynthesis. Amino acid supplementation experiments revealed that leucine specifically enhanced 3,4-AHBA production by strain KT01. Taken together, these results suggest that sweet sorghum juice is a potentially suitable feedstock for 3,4-AHBA production by recombinant C. glutamicum.
Novel benzene ring biosynthesis from C(3) and C(4) primary metabolites by two enzymes.[Pubmed:17003031]
J Biol Chem. 2006 Dec 1;281(48):36944-51.
The shikimate pathway, including seven enzymatic steps for production of chorismate via shikimate from phosphoenolpyruvate and erythrose-4-phosphate, is common in various organisms for the biosynthesis of not only aromatic amino acids but also most biogenic benzene derivatives. 3-Amino-4-hydroxybenzoic acid (3,4-AHBA) is a benzene derivative serving as a precursor for several secondary metabolites produced by Streptomyces, including grixazone produced by Streptomyces griseus. Our study on the biosynthesis pathway of grixazone led to identification of the biosynthesis pathway of 3,4-AHBA from two primary metabolites. Two genes, griI and griH, within the grixazone biosynthesis gene cluster were found to be responsible for the biosynthesis of 3,4-AHBA; the two genes conferred the in vivo production of 3,4-AHBA even on Escherichia coli. In vitro analysis showed that GriI catalyzed aldol condensation between two primary metabolites, l-aspartate-4-semialdehyde and dihydroxyacetone phosphate, to form a 7-carbon product, 2-amino-4,5-dihydroxy-6-one-heptanoic acid-7-phosphate, which was subsequently converted to 3,4-AHBA by GriH. The latter reaction required Mn(2+) ion but not any cofactors involved in reduction or oxidation. This pathway is independent of the shikimate pathway, representing a novel, simple enzyme system responsible for the synthesis of a benzene ring from the C(3) and C(4) primary metabolites.
Interaction of mushroom tyrosinase with aromatic amines, o-diamines and o-aminophenols.[Pubmed:15279888]
Biochim Biophys Acta. 2004 Aug 4;1673(3):170-7.
3-Amino-L-tyrosine was found to be a substrate of mushroom tyrosinase, contrary to what had previously been reported in the literature. A series of amino derivatives of benzoic acid were tested as substrates and inhibitors of the enzyme. 3-Amino-4-hydroxybenzoic acid, 4-amino-3-hydroxybenzoic acid and 3,4-diaminobenzoic acid were oxidized by this enzyme, as previously reported for Neurospora crassa tyrosinase, but 4-aminobenzoic acid and 3-aminobenzoic acid were not. Interestingly, 3-Amino-4-hydroxybenzoic acid was oxidized five times faster than 4-amino-3-hydroxybenzoic acid, confirming the importance of proton transfer from the hydroxyl group at C-4 position. All compounds inhibited the monophenolase activity but their effect on the diphenolase activity was small or negligible. 3-Amino-4-hydroxybenzoic acid was a stronger inhibitor than 4-amino-3-hydroxybenzoic acid, indicating their different binding affinity to the oxy form of the enzyme. Both, however, were weaker inhibitors than 3-amino-L-tyrosine, 4-methoxy-o-phenylenediamine and 3,4-diaminobenzoic acid, which was the strongest inhibitor from among the compounds tested. These results show that the relative positioning of the amino group and the hydroxy group in o-aminophenols with respect to the side chain is important both for binding to the dicopper center and for catalysis.


