TravoprostCAS# 157283-68-6 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

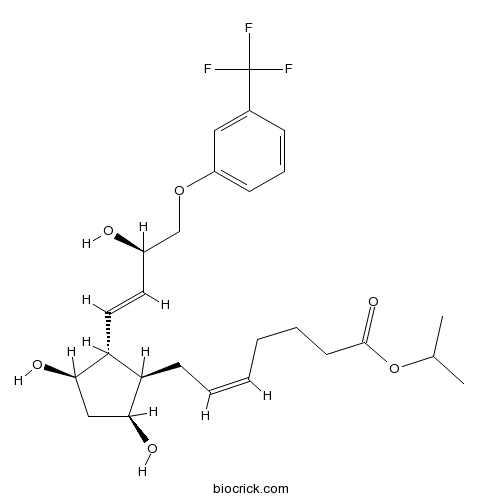
| Cas No. | 157283-68-6 | SDF | Download SDF |
| PubChem ID | 5282226 | Appearance | Powder |
| Formula | C26H35F3O6 | M.Wt | 500.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 41.67 mg/mL (83.25 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | propan-2-yl (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]but-1-enyl]cyclopentyl]hept-5-enoate | ||
| SMILES | CC(C)OC(=O)CCCC=CCC1C(CC(C1C=CC(COC2=CC=CC(=C2)C(F)(F)F)O)O)O | ||
| Standard InChIKey | MKPLKVHSHYCHOC-AHTXBMBWSA-N | ||
| Standard InChI | InChI=1S/C26H35F3O6/c1-17(2)35-25(33)11-6-4-3-5-10-21-22(24(32)15-23(21)31)13-12-19(30)16-34-20-9-7-8-18(14-20)26(27,28)29/h3,5,7-9,12-14,17,19,21-24,30-32H,4,6,10-11,15-16H2,1-2H3/b5-3-,13-12+/t19-,21-,22-,23+,24-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | FP prostaglandin receptor agonist. Exhibits submicromolar affinity for DP, EP, IP and TP receptors. Stimulates intracellular Ca2+ mobilization. Ocular hypotensive agent. |

Travoprost Dilution Calculator

Travoprost Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9978 mL | 9.989 mL | 19.978 mL | 39.956 mL | 49.9451 mL |
| 5 mM | 0.3996 mL | 1.9978 mL | 3.9956 mL | 7.9912 mL | 9.989 mL |
| 10 mM | 0.1998 mL | 0.9989 mL | 1.9978 mL | 3.9956 mL | 4.9945 mL |
| 50 mM | 0.04 mL | 0.1998 mL | 0.3996 mL | 0.7991 mL | 0.9989 mL |
| 100 mM | 0.02 mL | 0.0999 mL | 0.1998 mL | 0.3996 mL | 0.4995 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Travoprost is used to treat glaucoma and ocular hypertension.
- ML-323
Catalog No.:BCC4313
CAS No.:1572414-83-5
- Bosentan Hydrate
Catalog No.:BCC4494
CAS No.:157212-55-0
- 3-Amino-5-phenylpyrazole
Catalog No.:BCC8616
CAS No.:1572-10-7
- Harzianic acid
Catalog No.:BCN1838
CAS No.:157148-06-6
- Noopept
Catalog No.:BCC1804
CAS No.:157115-85-0
- Ajuforrestin A
Catalog No.:BCN8008
CAS No.:157110-18-4
- 3-Amino-4-hydroxybenzoic acid
Catalog No.:BCC8610
CAS No.:1571-72-8
- Lipedoside B-III
Catalog No.:BCC8201
CAS No.:157085-48-8
- SNAP 5089
Catalog No.:BCC7350
CAS No.:157066-77-8
- U 89843A
Catalog No.:BCC7466
CAS No.:157013-85-9
- 5,7-Dihydroxy-3,4',8-trimethoxyflavone
Catalog No.:BCN1551
CAS No.:1570-09-8
- H-D-Arg-OH
Catalog No.:BCC2868
CAS No.:157-06-2
- DMP 777
Catalog No.:BCC1534
CAS No.:157341-41-8
- MAP4
Catalog No.:BCC6758
CAS No.:157381-42-5
- Bacoside A3
Catalog No.:BCC8128
CAS No.:157408-08-7
- Taxuspine B
Catalog No.:BCN6938
CAS No.:157414-05-6
- Coronarin D methyl ether
Catalog No.:BCN1705
CAS No.:157528-81-9
- BTS
Catalog No.:BCC5425
CAS No.:1576-37-0
- (S)-SNAP 5114
Catalog No.:BCC7117
CAS No.:157604-55-2
- Boc-Ala-OH
Catalog No.:BCC3047
CAS No.:15761-38-3
- Boc-Pro-OH
Catalog No.:BCC3435
CAS No.:15761-39-4
- NSC 632839 hydrochloride
Catalog No.:BCC2088
CAS No.:157654-67-6
- Prenylpiperitol
Catalog No.:BCN1706
CAS No.:157659-20-6
- 7-Xylosyl-10-deacetylbaccatin III
Catalog No.:BCN7668
CAS No.:157664-03-4
The effects of selective laser trabeculoplasty and travoprost on circadian intraocular pressure fluctuations: A randomized clinical trial.[Pubmed:28178150]
Medicine (Baltimore). 2017 Feb;96(6):e6047.
BACKGROUND: To compare the effect of selective laser trabeculoplasty (SLT) and Travoprost on 24-hour IOP fluctuations in primary open-angle glaucoma (POAG) and normal-tension glaucoma (NTG). METHODS: Sixty eyes were included. Sixteen and 14 eyes of POAG patients were randomized to receive 360 degrees SLT or 0.004% Travoprost, respectively. Fourteen and 16 eyes of NTG patients were randomized to receive either SLT or Travoprost, respectively. The 24-hour IOP data were collected before treatment and 6 to 8 weeks after treatment. IOP was measured at 2 hours intervals in the sitting position during daytime (9 AM to 7 PM) and in the supine position during nighttime (9 PM to 7 AM). Main outcome measure was the percentage of eyes that achieved posttreatment 24-hour IOP fluctuations <3 mm Hg. Success in fluctuation reduction was defined as at least a 50% reduction in these fluctuations. RESULTS: Fifty-eight eyes were analyzed. Overall, eyes in the SLT and the Travoprost groups achieved a significant reduction in IOP compared with the baseline IOP values (-3.7 mm Hg [P = 0.002] vs -4.1 mm Hg [P < 0.001], respectively). There was no significant difference in IOP reduction in both groups according to type of glaucoma. During the diurnal period, 100% of POAG eyes in the Travoprost group achieved posttreatment IOP fluctuations <3 mm Hg, and 87% of eyes in the SLT group achieved the same level of fluctuations (P < 0.001). Ninety-six percent of NTG eyes in the Travoprost group, and 82% of eyes in the SLT group had IOP fluctuations <3 mm Hg (P = 0.01). Success in fluctuation reduction was 75% and 92% for the SLT and Travoprost groups, respectively (P = 0.005). The effect of Travoprost on IOP reduction in POAG and NTG patients was significant both during the daytime and the nighttime, while the SLT's effect was significant only during the nighttime. CONCLUSIONS: Both Travoprost and SLT can significantly reduce the IOP in patients with POAG and NTG. Based on habitual positions, Travoprost better controls IOP fluctuations than SLT, especially during the daytime.
Efficacy and Tolerability of Travoprost 0.004%/Timolol 0.5% Fixed-Dose Combination for the Treatment of Primary Open-Angle Glaucoma or Ocular Hypertension Inadequately Controlled with Beta-Blocker Monotherapy.[Pubmed:28239491]
J Ophthalmol. 2017;2017:1917570.
Objective. To evaluate the efficacy and tolerability of Travoprost 0.004%/timolol 0.5% fixed-dose combination (TTFC) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) inadequately controlled on beta-blocker monotherapy. Methods. In this phase IV, open-label study, 156 patients on beta-blocker monotherapy with mean intraocular pressure (IOP) between 18 and 32 mmHg were randomized (no washout period) to receive TTFC for 8 weeks (TTFC group) or to continue beta-blocker monotherapy for 4 weeks followed by TTFC for the remaining 4 weeks (beta-blocker group). Results. The mean IOP (+/-standard deviation) at baseline in the TTFC and beta-blocker groups was 22.5 +/- 2.5 mmHg and 22.2 +/- 2.3 mmHg, respectively, and at weeks 4 and 8, was 16.7 +/- 3.1 mmHg and 16.1 +/- 3.1 mmHg, respectively, in TTFC group and 21.1 +/- 3.1 mmHg and 16.1 +/- 2.8 mmHg, respectively, in the beta-blocker group. There was a significant least squares mean difference between TTFC and beta-blocker in 8 a.m. IOP at week 4 (-4.6 mmHg; one-sided 95% confidence interval [-inf, -3.9]; p < 0.0001 [primary endpoint]); the upper bound of the 95% confidence interval was within the prespecified limit (<0). Both treatments were well tolerated. Conclusion. Superior IOP control was achieved with TTFC in patients with OAG or OHT previously uncontrolled with beta-blockers. No new safety findings were identified. This trial is registered with ClinicalTrials.gov NCT02003391.
Fixed combination of travoprost and timolol maleate reduces intraocular pressure in Japanese patients with primary open-angle glaucoma or ocular hypertension: analysis by prostaglandin analogue.[Pubmed:28053501]
Clin Ophthalmol. 2016 Dec 20;11:55-61.
BACKGROUND: We have shown a decrease in mean intraocular pressure (IOP) by switching to Travoprost/timolol fixed combination (TTFC) in subjects receiving prostaglandin analogue (PGA) monotherapy and requiring additional medication in a previous report. For analyzing factors affecting IOP reduction, baseline IOP and preceding PGA were selected as statistically and clinically significant factors. In this report, we examine IOP-lowering effect and adverse drug reactions by preceding PGA. METHODS: Patients with primary open angle glaucoma or ocular hypertension who received monotherapy with one of four PGAs (Travoprost, latanoprost, tafluprost, or bimatoprost) for at least 3 months at 26 institutions and were determined to require additional medication by their primary physician were included. IOP reduction and adverse events were examined at 4, 8, and 12 weeks for each of four PGAs after switching to TTFC. RESULTS: In total, 157 patients who could be followed up for at least 4 weeks after switching to TTFC were included in the efficacy analysis. Multiple regression analysis was performed, and baseline IOP and PGA were found to be significant factors to IOP reduction. IOP reduction at week 12, adjusted with the regression model, was -3.5, -1.8, and -1.4 mmHg in the tafluprost, latanoprost, and Travoprost groups, whereas it was -0.5 mmHg in the bimatoprost group. Along with differences in baseline IOP between groups, an IOP-lowering effect of >1 mmHg was noted in the tafluprost, latanoprost, and Travoprost groups after the switch. IOP was maintained at 13.8-14.8 mmHg throughout the follow-up period. No serious adverse events or noteworthy issues were observed in any group after the switch. CONCLUSION: Clinically significant IOP-reducing effects of TTFC were observed in the latanoprost, Travoprost, and tafluprost groups when switching from each PGA monotherapy, while there were some differences in effects between groups, with minimal safety concerns.
Efficacy of two trabecular micro-bypass stents combined with topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 3-year follow-up.[Pubmed:28352151]
Clin Ophthalmol. 2017 Mar 15;11:523-528.
PURPOSE: To evaluate the long-term intraocular pressure (IOP)-lowering effect and safety parameters following treatment with two trabecular micro-bypass stents and topical prostaglandin in phakic eyes with open-angle glaucoma (OAG) not controlled on two preoperative medications. METHODS: This prospective, single-arm, unmasked study enrolled 39 qualified phakic eyes with OAG not controlled on 2 medications, preoperative medicated IOP of 18-30 mmHg, and IOP following medication washout of 22-38 mmHg. Two trabecular micro-bypass stents were implanted as a standalone procedure, and Travoprost was started on postoperative day 1. Evaluations included IOP, best-corrected visual acuity, medication use, fundus and slit-lamp examinations, visual field, cup:disc ratio, central corneal thickness, and ocular complications. Data through 18 months were summarized previously. Thirty-seven of the original 39 subjects have been followed for 3 years postoperatively; follow-up is continuing for 5 years. RESULTS: At 3 years postoperative, 97% of eyes had achieved an IOP reduction of >/=20% from baseline with a reduction of 1 medication. Eighty-six percent of eyes had IOP of Travoprost in phakic OAG eyes uncontrolled on 2 preoperative medications. These findings demonstrate the long-term performance and safety of trabecular bypass stents in combination with topical prostaglandin for OAG patients.


