Boc-Ala-OHCAS# 15761-38-3 |
Quality Control & MSDS
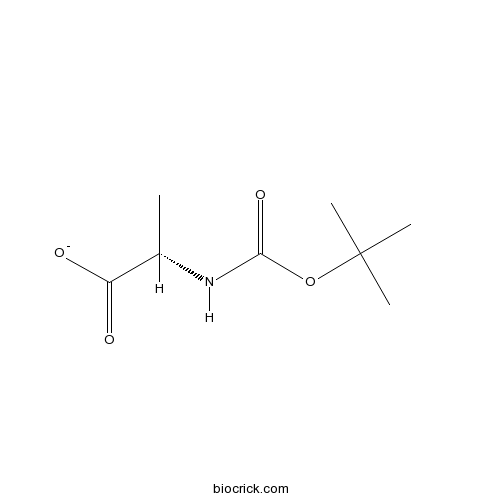
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15761-38-3 | SDF | Download SDF |
| PubChem ID | 7005102 | Appearance | Powder |
| Formula | C8H15NO4 | M.Wt | 189.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | (2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoate | ||
| SMILES | CC(C(=O)[O-])NC(=O)OC(C)(C)C | ||
| Standard InChIKey | QVHJQCGUWFKTSE-YFKPBYRVSA-M | ||
| Standard InChI | InChI=1S/C8H15NO4/c1-5(6(10)11)9-7(12)13-8(2,3)4/h5H,1-4H3,(H,9,12)(H,10,11)/p-1/t5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Ala-OH Dilution Calculator

Boc-Ala-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2854 mL | 26.4271 mL | 52.8541 mL | 105.7082 mL | 132.1353 mL |
| 5 mM | 1.0571 mL | 5.2854 mL | 10.5708 mL | 21.1416 mL | 26.4271 mL |
| 10 mM | 0.5285 mL | 2.6427 mL | 5.2854 mL | 10.5708 mL | 13.2135 mL |
| 50 mM | 0.1057 mL | 0.5285 mL | 1.0571 mL | 2.1142 mL | 2.6427 mL |
| 100 mM | 0.0529 mL | 0.2643 mL | 0.5285 mL | 1.0571 mL | 1.3214 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Ala-OH
- (S)-SNAP 5114
Catalog No.:BCC7117
CAS No.:157604-55-2
- BTS
Catalog No.:BCC5425
CAS No.:1576-37-0
- Coronarin D methyl ether
Catalog No.:BCN1705
CAS No.:157528-81-9
- Taxuspine B
Catalog No.:BCN6938
CAS No.:157414-05-6
- Bacoside A3
Catalog No.:BCC8128
CAS No.:157408-08-7
- MAP4
Catalog No.:BCC6758
CAS No.:157381-42-5
- DMP 777
Catalog No.:BCC1534
CAS No.:157341-41-8
- Travoprost
Catalog No.:BCC5189
CAS No.:157283-68-6
- ML-323
Catalog No.:BCC4313
CAS No.:1572414-83-5
- Bosentan Hydrate
Catalog No.:BCC4494
CAS No.:157212-55-0
- 3-Amino-5-phenylpyrazole
Catalog No.:BCC8616
CAS No.:1572-10-7
- Harzianic acid
Catalog No.:BCN1838
CAS No.:157148-06-6
- Boc-Pro-OH
Catalog No.:BCC3435
CAS No.:15761-39-4
- NSC 632839 hydrochloride
Catalog No.:BCC2088
CAS No.:157654-67-6
- Prenylpiperitol
Catalog No.:BCN1706
CAS No.:157659-20-6
- 7-Xylosyl-10-deacetylbaccatin III
Catalog No.:BCN7668
CAS No.:157664-03-4
- Perifosine
Catalog No.:BCC3673
CAS No.:157716-52-4
- Gentiournoside D
Catalog No.:BCN7855
CAS No.:157722-21-9
- Gypenoside A
Catalog No.:BCN8459
CAS No.:157752-01-7
- 5-Nonyloxytryptamine oxalate
Catalog No.:BCC6839
CAS No.:157798-13-5
- 5-Benzimidazolecarboxylic acid
Catalog No.:BCC8739
CAS No.:15788-16-6
- (Z)-2-decenoic acid
Catalog No.:BCC1295
CAS No.:15790-91-7
- Chitinase-IN-1
Catalog No.:BCC5533
CAS No.:1579991-61-9
- Chitinase-IN-2
Catalog No.:BCC5534
CAS No.:1579991-63-1
Rapid approach to 3,5-disubstituted 1,4-benzodiazepines via the photo-fries rearrangement of anilides.[Pubmed:17109551]
J Org Chem. 2006 Nov 24;71(24):9217-20.
Different anilides derived from carboxylic acids and substituted anilines have been submitted to the photochemically induced Fries rearrangement giving the corresponding o-amino phenones under conditions that are compatible with the presence of acid-labile groups (such as N-Boc or TBDMSO) on R1 and R3. These compounds, not easily obtained in other ways, are useful building blocks for the preparation of benzocondensated heterocycles. After coupling with N-Boc amino acids and TFA-mediated deprotection, the products cyclized to the corresponding 3,5-disubstituted 1,4-benzodiazepin-2-ones, privileged structures predominantly active in the central nervous system. The same results were obtained by coupling with N-Cbz-protected alpha-amino acids followed by microwave assisted hydrogenolysis. When the Fries rearrangement was carried out on the anilide derived from N-Boc-Ala-OH and the further coupling done with N-Cbz-(OMe)Asp-OH, the formed benzodiazepines could be inserted in a peptide chain for the preparation of conformationally constrained peptidomimetics.
Radical acylation of L-lysine derivatives and L-lysine-containing peptides by peroxynitrite-treated diacetyl and methylglyoxal.[Pubmed:24328571]
Free Radic Res. 2014 Mar;48(3):357-70.
Highly electrophilic alpha-dicarbonyls such as diacetyl, methylglyoxal, 3-deoxyglucosone, and4,5-dioxovaleric acid have been characterized as secondary catabolites that can aggregate proteins and form DNA nucleobase adducts in several human maladies, including Alzheimer's disease, rheumatoid arthritis, diabetes, sepsis, renal failure, and respiratory distress syndrome. In vitro, diacetyl and methylglyoxal have also been shown to rapidly add up the peroxynitrite anion (k2 ~ 10(4)-10(5) M(-1) s(-1)), a potent biological nucleophile, oxidant and nitrosating agent, followed by carbon chain cleavage to carboxylic acids via acetyl radical intermediate that can modify amino acids. In this study, we used the amino acid derivatives Ac-Lys-OMe and Z-Lys-OMe and synthesized the tetrapeptides H-KALA-OH, Ac-KALA-OH, and H-K(Boc)ALA-OH to reveal the preferential Lys amino group targeted by acyl radical generated by the alpha-dicarbonyl/peroxynitrite system. The pH profiles of the reactions are bell-shaped, peaking at approximately 7.5; hence, they are close to the pKa values of ONOOH and of the catalytic H2PO4(-) anion. RP-HPLC and ESI-MS analyses of reaction products confirmed (alpha)N- and ()N-acetylation of Lys by diacetyl as well as acetylation and formylation by methylglyoxal, with preference for the alpha-amino group. These data suggest the possibility of radical acylation of proteins in epigenetic processes, where enzymatic acetylation of these biomolecules is a well-documented event, recently reported to be as critical to the cell cycle as phosphorylation. Also noteworthy is the observed formylation of L-Lys containing peptides by methylglyoxal never reported to occur in amino acid residues of peptides and proteins.
Evaluation of carbodiimides using a competition method.[Pubmed:9230479]
J Pept Sci. 1997 Mar-Apr;3(2):141-4.
A competitive reaction of activated Boc-Ala-OH and Boc-Phe-OH with H-Leu-resin has been developed for assessing the relative efficiencies of different carbodiimides. This allowed a comparison of the efficiency of the carbodiimides N,N'-dicyclohexylcarbodiimide,N,N'-diisopropylcarbodiimide, N-tert-butyl-N'-methylcarbodiimide and N-tert-butyl-N'-ethylcarbodiimide. Comparable results were obtained when these reagents were used for the preformation of symmetrical anhydrides or of 1-hydroxybenzotriazole esters in situ. Differential incorporation was observed when asymmetrical carbodiimides were used for peptide bond formation by the direct carbodiimide procedure.


