3'-HydroxypterostilbeneCAS# 475231-21-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 475231-21-1 | SDF | Download SDF |
| PubChem ID | 10038868.0 | Appearance | Powder |
| Formula | C16H16O4 | M.Wt | 272.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
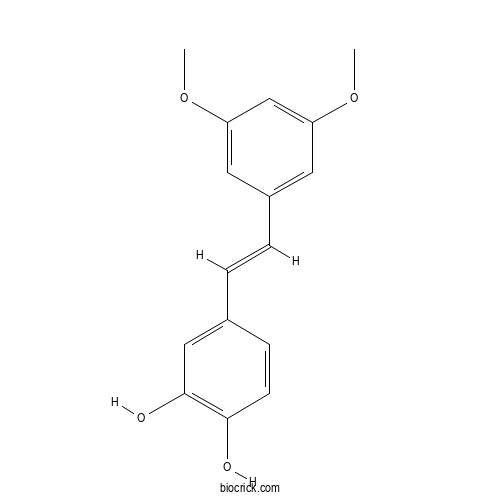
| Chemical Name | 4-[(E)-2-(3,5-dimethoxyphenyl)ethenyl]benzene-1,2-diol | ||
| SMILES | COC1=CC(=CC(=C1)C=CC2=CC(=C(C=C2)O)O)OC | ||
| Standard InChIKey | UQRBFXIUUDJHSN-ONEGZZNKSA-N | ||
| Standard InChI | InChI=1S/C16H16O4/c1-19-13-7-12(8-14(10-13)20-2)4-3-11-5-6-15(17)16(18)9-11/h3-10,17-18H,1-2H3/b4-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3'-Hydroxypterostilbene Dilution Calculator

3'-Hydroxypterostilbene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6724 mL | 18.3621 mL | 36.7242 mL | 73.4484 mL | 91.8105 mL |
| 5 mM | 0.7345 mL | 3.6724 mL | 7.3448 mL | 14.6897 mL | 18.3621 mL |
| 10 mM | 0.3672 mL | 1.8362 mL | 3.6724 mL | 7.3448 mL | 9.1811 mL |
| 50 mM | 0.0734 mL | 0.3672 mL | 0.7345 mL | 1.469 mL | 1.8362 mL |
| 100 mM | 0.0367 mL | 0.1836 mL | 0.3672 mL | 0.7345 mL | 0.9181 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Melezitose
Catalog No.:BCX0728
CAS No.:597-12-6
- 6-Methoxy-2-[2-(3'-methoxyphenyl)ethyl]chromone
Catalog No.:BCX0727
CAS No.:84294-88-2
- 6-Methoxy-2-(2-phenylethyl)chromone
Catalog No.:BCX0726
CAS No.:84294-89-3
- (+)-Rabdosiin
Catalog No.:BCX0725
CAS No.:263397-69-9
- Neoanhydropodophyllol
Catalog No.:BCX0724
CAS No.:62287-47-2
- Melibiose
Catalog No.:BCX0723
CAS No.:585-99-9
- Rabdosiin
Catalog No.:BCX0722
CAS No.:119152-54-4
- 7-Ketodeoxycholic acid
Catalog No.:BCX0721
CAS No.:911-40-0
- Ligupurpuroside D
Catalog No.:BCX0720
CAS No.:1194056-35-3
- D-Lyxose
Catalog No.:BCX0719
CAS No.:1114-34-7
- Anthrone
Catalog No.:BCX0718
CAS No.:90-44-8
- limocitrin -3-O-rutinoside
Catalog No.:BCX0717
CAS No.:79384-27-3
- 6,7-Dimethoxy-2-[2-(4'-methoxyphenyl)ethyl]chromone
Catalog No.:BCX0730
CAS No.:117596-92-6
- Maltoheptaose
Catalog No.:BCX0731
CAS No.:34620-78-5
- Fructo-oligosaccharide DP14/GF13
Catalog No.:BCX0732
CAS No.:137405-38-0
- (9Z,12Z,15Z)-N-[(3-Methoxyphenyl)methyl]-9,12,15-octadecatrienamide
Catalog No.:BCX0733
CAS No.:883715-23-9
- Fructo-oligosaccharide DP10/GF9
Catalog No.:BCX0734
CAS No.:118150-64-4
- Glucosinalbin
Catalog No.:BCX0735
CAS No.:19253-84-0
- Fructo-oligosaccharide DP9/GF8
Catalog No.:BCX0736
CAS No.:143625-74-5
- Fructo-oligosaccharide DP13/GF12
Catalog No.:BCX0737
CAS No.:137405-37-9
- (-)-Myrtenal
Catalog No.:BCX0738
CAS No.:18486-69-6
- 6-Methoxyldihydrochelerythrine
Catalog No.:BCX0739
CAS No.:21080-31-9
- Lentinan
Catalog No.:BCX0740
CAS No.:37339-90-5
- Edgeworoside C
Catalog No.:BCX0741
CAS No.:126221-40-7
3'-Hydroxypterostilbene Potently Suppresses Tumor Growth via Inhibiting the Activation of the JAK2/STAT3 Pathway in Ovarian Clear Cell Carcinoma.[Pubmed:37876143]
Mol Nutr Food Res. 2024 Jan;68(1):e2300108.
SCOPE: Ovarian clear cell carcinoma (OCCC) is a subtype of epithelial ovarian cancer (EOC) that is associated with higher interleukin-6 (IL-6) levels, and suppression of the Janus kinase 2/Signal transducer and activator of transription 3 (JAK2/STAT3) pathway may contribute to the suppression of this cancer. This study aims to compare the anti-cancer effect of pterostilbene (PSB) and 2'- and 3'-hydroxypterostilbene (2HPSB and 3HPSB, respectively) on the JAK2/STAT3 pathway. METHODS AND RESULTS: In vitro experiments with the OCCC cell line TOV21G and a xenograft nude mouse model are used to achieve the study aims. The results showed that 3HPSB has the greatest anti-proliferative and pro-apoptotic effects of the three compounds studied. Activation of the JAK2/STAT3 pathway and the nuclear translocation of STAT3 are effectively inhibited by 3HPSB and PSB. Both 3HPSB and PSB can effectively suppress tumor growth, which is mediated by the inhibition of JAK2/STAT3 phosphorylation. CONCLUSION: This is the first study to compare the efficacy of PSB, 3HPSB, and the newly identified compound 2HPSB regarding ovarian cancer. Moreover, targeting JAK2/STAT3 is shown to be a potentially effective strategy for OCCC treatment. This study is expected to provide new insights into the potential of the abovementioned phytochemicals for development as adjuvants for cancer treatment in the future.
Piceatannol and 3'-Hydroxypterostilbene Alleviate Inflammatory Bowel Disease by Maintaining Intestinal Epithelial Integrity and Regulating Gut Microbiota in Mice.[Pubmed:36688924]
J Agric Food Chem. 2023 Feb 1;71(4):1994-2005.
Inflammatory bowel disease has become a significant health concern across the globe, causing frequent and long-term harm to the digestive system. This study evaluated the effect of piceatannol (PIC) and 3'-hydroxypterostilbene (HPSB) on dextran sulfate sodium (DSS)-induced colitis in mice and investigated whether their effects are exerted through the amelioration of gut barrier dysfunction to reduce the severity of colitis. The findings showed that both PIC and HPSB attenuated inflammation by inhibiting the TNF-alpha/NF-kappaB/MLC pathway and reducing NLRP3 inflammasome activation. However, PIC was comparably effective in modulating tight junctions. The results may be attributed to the effect of PIC on reducing cell apoptosis-associated protein expression, including Bax/Bcl-2 and caspase-3 activation. Furthermore, microbiota analysis revealed that both PIC and HPSB increased representative probiotic species, including Akkermansiaceae and Lactobacillus intestinalis, and exhibited inhibitory effects on several bacterial species (Spiroplasmataceae and Acholeplasmataceae). Based on linear discriminant analysis effect size, butyrate-producing bacteria were identified as a biomarker in the PIC group. Overall, the results demonstrated that PIC repressed inflammation, inhibited cell apoptosis, and regulated microbiota composition. Consequently, PIC is more effective in maintaining gut barrier integrity than HPSB, and it is a promising ingredient in the development of functional food for colitis prevention.
Phase I Metabolism of Pterostilbene, a Dietary Resveratrol Derivative: Metabolite Identification, Species Differences, Isozyme Contribution, and Further Bioactivation.[Pubmed:36538288]
J Agric Food Chem. 2023 Jan 11;71(1):331-346.
Pterostilbene (PTE), a dietary derivative of resveratrol, displayed pleiotropic health-promoting activities. This study aimed to explore the metabolic profiles and species differences of the phase I metabolism of PTE and to investigate subsequent detoxification after PTE bioactivation. PTE was found to be biotransformed to two pharmacologically active metabolites, pinostilbene and 3'-hydroxypterostilbene, in vivo and in vitro with substantial species differences. Human CYP1A2 was proved to be mainly responsible for the demethylation and 3'-hydroxylation of PTE, with its contribution to a demethylation of 94.5% and to a 3'-hydroxylation of 97.9%. An in vitro glutathione trapping experiment revealed the presence of an ortho-quinone intermediate formed by further oxidation of 3'-hydroxypterostilbene. Human glutathione S-transferase isoforms A2, T1, and A1 inactivated the ortho-quinone intermediate by catalyzing glutathione conjugation, implicating a potential protective pathway against PTE bioactivation-derived toxicity. Overall, this study provided a comprehensive view of PTE phase I metabolism and facilitated its further development as a promising nutraceutical.
Pterostilbene and Its Derivative 3'-Hydroxypterostilbene Ameliorated Nonalcoholic Fatty Liver Disease Through Synergistic Modulation of the Gut Microbiota and SIRT1/AMPK Signaling Pathway.[Pubmed:35416649]
J Agric Food Chem. 2022 Apr 27;70(16):4966-4980.
Nonalcoholic fatty liver disease (NAFLD) is a recent chronic liver disease common in many developed countries and is closely associated with metabolic syndrome, such as obesity and insulin resistance. The present study was performed to investigate the effects of pterostilbene (Pt) and its derivative 3'-hydroxypterostilbene (OHPt) on free fatty acids (FFA)-induced lipid accumulation in HepG2 cells and high-fat diet (HFD)-induced NAFLD in C57BL/6J mice. The results showed that Pt and OHPt significantly ameliorated FFA-induced steatosis in HepG2 cells and enhanced lipolysis through the upregulation of SIRT1/AMPK and insulin signaling pathways. In the in vivo study, Pt and OHPt treatment resulted in reduced hepatic lipid droplets accumulation. The data showed that Pt and OHPt upregulated the SIRT1/AMPK pathway and subsequently downregulated the protein expression of SREBP-1 to activate fatty acid (FA) beta-oxidation to inhibit FA synthesis. Pt and OHPt administration activated the insulin signaling pathway and further ameliorated the insulin resistance and liver function in the HFD-fed mice. Furthermore, Pt and OHPt markedly increased the numbers of Oscillospira and decreased the numbers of Allobaculum, Phascolarctobacterium, and Staphylococcus compared with those in the HFD group. These robust results indicate that Pt and OHPt are able to possess potential health benefits in improving insulin resistance and hepatic steatosis by promoting healthy populations or abundances of considered vital microbiota. Besides, OHPt is more effective than Pt, which might have promising chemotherapeutic effects for future clinical application.
3'-Hydroxypterostilbene Inhibits 7,12-Dimethylbenz[a]anthracene (DMBA)/12-O-Tetradecanoylphorbol-13-Acetate (TPA)-Induced Mouse Skin Carcinogenesis.[Pubmed:33310310]
Phytomedicine. 2021 Jan;81:153432.
BACKGROUND: A natural pterostilbene analogue isolated from the herb Sphaerophysa salsula, 3'-hydroxypterostilbene (HPSB), exhibits antiproliferative activity in several cancer cell lines; however, the inhibitory effects of HPSB on skin carcinogenesis remains unclear. PURPOSE: The aim of this study was to evaluate the inhibitory effects of HPSB on two-stage skin carcinogenesis in mice and its potential mechanism. STUDY DESIGN AND METHODS: This study investigated the anti-inflammatory and anti-tumor effects of HPSB in the 12-O-tetradecanoylphorbol-13-acetate (TPA)-stimulated acute skin inflammation and 7,12-dimethylbenz[a]anthracene (DMBA)/TPA-induced two-stage skin carcinogenesis model. In addition, the effects of HPSB on the modulation of the phase I and phase II metabolizing enzymes in the DMBA-induced HaCaT cell model were investigated. RESULTS: The results provide evidence that topical treatment with HPSB significantly inhibits TPA-induced epidermal hyperplasia and leukocyte infiltration through the down-regulation of cyclooxygenase-2 (COX-2), matrix metalloprotein-9 (MMP-9), and ornithine decarboxylase (ODC) protein expression in mouse skin. Furthermore, HPSB suppresses DMBA/TPA-induced skin tumor incidence and multiplicity via the inhibition of proliferating cell nuclear antigen (PCNA), Cyclin B1 and cyclin-dependent kinase 1 (CDK1) expression in the two-stage skin carcinogenesis model. In addition, pretreatment with HPSB markedly reduces DMBA-induced cytochrome P450 1A1 (CYP1A1) and cytochrome P450 1B1 (CYP1B1) gene expression in human keratinocytes; however, HPSB does not significantly affect the gene expression of the phase II enzymes. CONCLUSION: This is the first study to show that topical treatment with HPSB prevents mouse skin tumorigenesis. Overall, our study suggests that natural HPSB may serve as a novel chemopreventive agent capable of preventing carcinogen activation and inflammation-associated tumorigenesis.
3'-Hydroxypterostilbene Potently Alleviates Obesity Exacerbated Colitis in Mice.[Pubmed:32316726]
J Agric Food Chem. 2020 May 13;68(19):5365-5374.
Epidemiological surveys show that obesity and the western diet increase the risk of colitis. Studies have also confirmed that the high-fat-diet (HFD) promoted the deterioration of colitis-related indicators in mice. Compared with stilbenoids, the results showed that 3'-hydroxypterostilbene (HPSB) was found to be the most effective inhibitor for the antiadipogenesis and anti-inflammation. However, its role in ameliorating obesity-promoted colitis is still unknown. We intend to investigate the protective effect and related molecular mechanisms of HPSB on HFD promoted dextran sodium sulfate (DSS)-induced colitis in mice. The results indicate that colitis in the HFD+DSS group tends to be more apparent in the DSS-only group, while feeding 0.025% of HPSB at different stages can improve the colitis induced by HFD+DSS. HPSB significantly reduced the levels of interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) induced by HFD+DSS in mice. Furthermore, the Western blotting revealed that the administration of HPSB significantly downregulated cyclooxygenase-2 (COX-2), plasmalemma vesicle-associated protein-1 (PV-1), and phospho-signal transducer and activator of transcription 3 (p-STAT3) expressions in HFD+DSS treated mice. Presented results reveal that HPSB is a novel functional agent capable of preventing HFD exacerbated colitis.
The relationship between structure and in vitro antistaphylococcal effect of plant-derived stilbenes.[Pubmed:30203690]
Acta Microbiol Immunol Hung. 2018 Dec 1;65(4):467-476.
Staphylococcus aureus is a major human pathogen that is responsible for both hospital- and community-acquired infections. Stilbenes are polyphenol compounds of plant origin known to possess a variety of pharmacological properties, such as antibacterial, antiviral, and antifungal effects. This study reports the in vitro growth-inhibitory potential of eight naturally occurring stilbenes against six standard strains and two clinical isolates of S. aureus, using a broth microdilution method, and expressing the results as minimum inhibitory concentrations (MICs). Pterostilbene (MICs = 32-128 mug/ml), piceatannol (MICs = 64-256 mug/ml), and pinostilbene (MICs = 128 mug/ml) are among the active compounds that possess the strongest activity against all microorganisms tested, followed by 3'-hydroxypterostilbene, isorhapontigenin, oxyresveratrol, and rhapontigenin with MICs 128-256 mug/ml. Resveratrol (MIC = 256 mug/ml) exhibited only weak inhibitory effect. Furthermore, structure-activity relationships were studied. Hydroxyl groups at ortho-position (B-3' and -4') played crucial roles for the inhibitory effect of hydroxystilbene piceatannol. Compounds with methoxy groups at ring A (3'-hydroxypterostilbene, pinostilbene, and pterostilbene) produced stronger effect against S. aureus than their analogues (isorhapontigenin and rhapontigenin) with methoxy groups at ring B. These findings provide arguments for further investigation of stilbenes as prospective leading structures for development of novel antistaphylococcal agents for topical treatment of skin infections.
3'-Hydroxypterostilbene Suppresses Colitis-Associated Tumorigenesis by Inhibition of IL-6/STAT3 Signaling in Mice.[Pubmed:29032686]
J Agric Food Chem. 2017 Nov 8;65(44):9655-9664.
3'-Hydroxypterostilbene (trans-3,5-dimethoxy-3',4'-hydroxystilbene) presents in Sphaerophysa salsula, Pterocarpus marsupium, and honey bee propolis and has been reported to exhibit several biological activities. Herein, we aimed to explore the chemopreventive effects of dietary 3'-hydroxypterostilbene and underlying molecular mechanisms on colitis-associated cancer using the azoxymethane (AOM)/dextran sodium sulfate (DSS) model. 3'-Hydroxypterostilbene administration effectively ameliorated the colon shortening and number of tumors in AOM/DSS-treated mice (3.2 +/- 1.2 of the high-dose treatment versus 13.8 +/- 5.3 of the AOM/DSS group, p < 0.05). Molecular analysis exhibited the anti-inflammatory activity of 3'-hydroxypterostilbene by a significant decrease in the levels of inducible nitric oxide synthase, cyclooxygenase-2, and interleukin-6 (IL-6) (p < 0.05). Moreover, dietary 3'-hydroxypterostilbene also significantly diminished IL-6/signal transducer and activator of transcription signaling and restored colonic suppressor of cytokine signaling 3 levels in the colonic tissue of mice (p < 0.05). Collectively, these results demonstrated for the first time the in vivo chemopreventive efficacy and molecular mechanisms of dietary 3'-hydroxypterostilbene against colitis-associated colonic tumorigenesis.
Biological actions and molecular effects of resveratrol, pterostilbene, and 3'-hydroxypterostilbene.[Pubmed:28911531]
J Food Drug Anal. 2017 Jan;25(1):134-147.
Stilbenes are a class of polyphenolic compounds, naturally found in a wide variety of dietary sources such as grapes, berries, peanuts, red wine, and some medicinal plants. There are several well-known stilbenes including trans-resveratrol, pterostilbene, and 3'-hydroxypterostilbene. The core chemical structure of stilbene compounds is 1,2-diphenylethylene. Recently, stilbenes have attracted extensive attention and interest due to their wide range of health-beneficial effects such as anti-inflammation, -carcinogenic, -diabetes, and -dyslipidemia activities. Moreover, accumulating in vitro and in vivo studies have reported that stilbene compounds act as inducers of multiple cell-death pathways such as apoptosis, cell cycle arrest, and autophagy for chemopreventive and chemotherapeutic agents in several types of cancer cells. The aim of this review is to highlight recent molecular findings and biological actions of trans-resveratrol, pterostilbene, and 3'-hydroxypterostilbene.
alpha-Glucosidase inhibitory effect of resveratrol and piceatannol.[Pubmed:28570943]
J Nutr Biochem. 2017 Sep;47:86-93.
Dietary polyphenols have been shown to inhibit alpha-glucosidase, an enzyme target of some antidiabetic drugs. Resveratrol, a polyphenol found in grapes and wine, has been reported to inhibit the activity of yeast alpha-glucosidase. This triggered our interest to synthesize analogs and determine their effect on mammalian alpha-glucosidase activity. Using either sucrose or maltose as substrate resveratrol, piceatannol and 3'-hydroxypterostilbene showed strong inhibition of mammalian alpha-glucosidase activity; pinostilbene, cis-desoxyrhapontigenin and trans-desoxyrhapontigenin had moderate inhibition. Compared to acarbose (IC(50) 3-13 mug/ml), piceatannol and resveratrol inhibited mammalian alpha-glucosidase to a lesser extent (IC(50) 14-84 and 111-120 mug/ml, respectively). 3'-Hydroxypterostilbene (IC(50) 105-302 mug/ml) was 23-35-fold less potent than acarbose. We investigated the effect of piceatannol and resveratrol on postprandial blood glucose response in high-fat-fed C57Bl/6 mice. Animals administered resveratrol (30 mg/kg body weight [BW]) or piceatannol (14 mg/kg BW) 60 min prior to sucrose or starch loading had a delayed absorption of carbohydrates, resulting in significant lowering of postprandial blood glucose concentrations, similar to the antidiabetic drug acarbose, while no significant effect was observed with the glucose-loaded animals. Our studies demonstrate that the dietary polyphenols resveratrol and piceatannol lower postprandial hyperglycemia and indicate that inhibition of intestinal alpha-glucosidase activity may be a potential mechanism contributing to their antidiabetic property.
Evaluation of 90 day repeated dose oral toxicity and reproductive/developmental toxicity of 3'-hydroxypterostilbene in experimental animals.[Pubmed:28257483]
PLoS One. 2017 Mar 3;12(3):e0172770.
3'-Hydroxypterostilbene (3'-HPT) is one of the active constituents of Sphaerophysa salsula and Pterocarpus marsupium. Despite many proposed therapeutic applications, the safety profile of 3'-HPT has not been established. The present work investigated 90 day repeated oral dose and reproductive (developmental) toxicity of 3'-HPT as a test substance in rats as per OECD guidelines. 90 day toxicity was conducted in sixty Sprague Dawley rats of each sex (120 rats), grouped into six dosage groups of 0 (control), 0 (control recovery), 20 (low dose), 80 (mid dose), 200 (high dose) and 200 (high dose recovery) mg/kg bwt/day (body weight/day) respectively. For the reproductive toxicity study forty Wistar rats of each sex (80 rats) divided into four dosage groups received 0 (vehicle control), 20 (low dose), 100 (mid dose) and 200 (high dose) mg/kg bwt/day of 3'-HPT respectively for a period of two weeks while pre-mating, mating, on the day before sacrifice, in females during pregnancy and four days of lactation period. Results showed no significant differences in body weight, food intake, absolute organ weight, haematology, with no adverse effects (toxicity) on biochemical values nor any abnormal clinical signs or behavioural changes were observed in any of the control/treatment groups, including reproductive and developmental parameters, gross and histopathological changes. In conclusion, the results suggested a No-Observed-Adverse-Effect-Level (NOAEL) of 200 mg/kg bwt/day in rats after oral administration, implying 3'-HPT did not exhibit any toxicity under the study conditions employed.
Pharmacologic Activities of 3'-Hydroxypterostilbene: Cytotoxic, Anti-Oxidant, Anti-Adipogenic, Anti-Inflammatory, Histone Deacetylase and Sirtuin 1 Inhibitory Activity.[Pubmed:26626255]
J Pharm Pharm Sci. 2015;18(4):713-27.
PURPOSE: Delineate the selected pharmacodynamics of a naturally occurring stilbene 3'-Hydroxypterostilbene. OBJECTIVE: Characterize for the first time the pharmacodynamics bioactivity in several in-vitro assays with relevant roles in heart disease, inflammation, cancer, and diabetes etiology and pathophysiology. METHODS: 3'-Hydroxypterostilbene was studied in in-vitro assays to identify possible bioactivity. RESULTS: 3'-Hydroxypterostilbene demonstrated anti-oxidant, anti-inflammatory, cytotoxic, anti-adipogenic, histone deacetylase, and sirtuin-1 inhibitory activity. CONCLUSIONS: The importance of understanding individual stilbene pharmacologic activities were delineated. Small changes in chemical structure of stilbene compounds result in significant pharmacodynamic differences. This article is open to POST-PUBLICATION REVIEW. Registered readers (see "For Readers") may comment by clicking on ABSTRACT on the issue's contents page.
Potent anti-cancer effect of 3'-hydroxypterostilbene in human colon xenograft tumors.[Pubmed:25389774]
PLoS One. 2014 Nov 12;9(11):e111814.
Here we report that 3'-hydroxypterostilbene (HPSB), a natural pterostilbene analogue, was more potent than pterostilbene against the growth of human cancer cells (COLO 205, HCT-116, and HT-29) with measured IC50 values of 9.0, 40.2, and 70.9 microM, respectively. We found that HPSB effectively inhibited the growth of human colon cancer cells by inducing apoptosis and autophagy. Autophagy occurred at an early stage and was observed through the formation of acidic vesicular organelles and microtubule-associated protein 1 light chain 3-II production. At the molecular levels, the results from western blot analysis showed that HPSB significantly down-regulated phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein kinases (MAPKs) signalings including decreased the phosphorylation of mammalian target of rapamycin (mTOR). Significant therapeutic effects were demonstrated in vivo by treating nude mice bearing COLO 205 tumor xenografts with HPSB (10 mg/kg i.p.). These inhibitory effects were accompanied by mechanistic down-regulation of the protein levels of cyclooxygenase-2 (COX-2), matrix metallopeptidase-9 (MMP-9), vascular endothelial growth factor (VEGF), and cyclin D1, as well as by the induction of apoptosis in colon tumors. Our findings suggest that HPSB could serve as a novel promising agent for colon cancer treatment.


