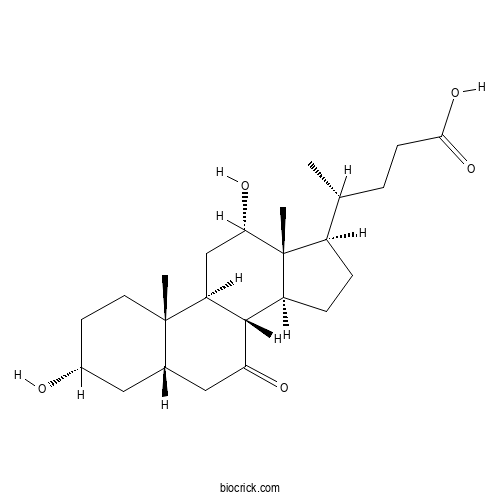
7-Ketodeoxycholic acidCAS# 911-40-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 911-40-0 | SDF | Download SDF |
| PubChem ID | 188292.0 | Appearance | Powder |
| Formula | C24H38O5 | M.Wt | 406.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4R)-4-[(3R,5S,8R,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethyl-7-oxo-1,2,3,4,5,6,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]pentanoic acid | ||
| SMILES | CC(CCC(=O)O)C1CCC2C1(C(CC3C2C(=O)CC4C3(CCC(C4)O)C)O)C | ||
| Standard InChIKey | RHCPKKNRWFXMAT-RRWYKFPJSA-N | ||
| Standard InChI | InChI=1S/C24H38O5/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26/h13-18,20,22,25,27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16-,17+,18+,20+,22+,23+,24-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

7-Ketodeoxycholic acid Dilution Calculator

7-Ketodeoxycholic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4597 mL | 12.2983 mL | 24.5966 mL | 49.1932 mL | 61.4915 mL |
| 5 mM | 0.4919 mL | 2.4597 mL | 4.9193 mL | 9.8386 mL | 12.2983 mL |
| 10 mM | 0.246 mL | 1.2298 mL | 2.4597 mL | 4.9193 mL | 6.1492 mL |
| 50 mM | 0.0492 mL | 0.246 mL | 0.4919 mL | 0.9839 mL | 1.2298 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.246 mL | 0.4919 mL | 0.6149 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ligupurpuroside D
Catalog No.:BCX0720
CAS No.:1194056-35-3
- D-Lyxose
Catalog No.:BCX0719
CAS No.:1114-34-7
- Anthrone
Catalog No.:BCX0718
CAS No.:90-44-8
- limocitrin -3-O-rutinoside
Catalog No.:BCX0717
CAS No.:79384-27-3
- Prunetrin
Catalog No.:BCX0716
CAS No.:154-36-9
- Salsolinol
Catalog No.:BCX0715
CAS No.:27740-96-1
- Bufotenidine
Catalog No.:BCX0714
CAS No.:487-91-2
- Epigallocatechin gallate octaacetate
Catalog No.:BCX0713
CAS No.:148707-39-5
- Oleandrin,anhydro-16-deacetyl-
Catalog No.:BCX0712
CAS No.:69549-58-2
- Pinolenic acid
Catalog No.:BCX0711
CAS No.:16833-54-8
- Digitoxigenin
Catalog No.:BCX0710
CAS No.:143-62-4
- Oleandrigenin
Catalog No.:BCX0709
CAS No.:465-15-6
- Rabdosiin
Catalog No.:BCX0722
CAS No.:119152-54-4
- Melibiose
Catalog No.:BCX0723
CAS No.:585-99-9
- Neoanhydropodophyllol
Catalog No.:BCX0724
CAS No.:62287-47-2
- (+)-Rabdosiin
Catalog No.:BCX0725
CAS No.:263397-69-9
- 6-Methoxy-2-(2-phenylethyl)chromone
Catalog No.:BCX0726
CAS No.:84294-89-3
- 6-Methoxy-2-[2-(3'-methoxyphenyl)ethyl]chromone
Catalog No.:BCX0727
CAS No.:84294-88-2
- Melezitose
Catalog No.:BCX0728
CAS No.:597-12-6
- 3'-Hydroxypterostilbene
Catalog No.:BCX0729
CAS No.:475231-21-1
- 6,7-Dimethoxy-2-[2-(4'-methoxyphenyl)ethyl]chromone
Catalog No.:BCX0730
CAS No.:117596-92-6
- Maltoheptaose
Catalog No.:BCX0731
CAS No.:34620-78-5
- Fructo-oligosaccharide DP14/GF13
Catalog No.:BCX0732
CAS No.:137405-38-0
- (9Z,12Z,15Z)-N-[(3-Methoxyphenyl)methyl]-9,12,15-octadecatrienamide
Catalog No.:BCX0733
CAS No.:883715-23-9
Enhanced protein-metabolite correlation analysis: To investigate the association between Staphylococcus aureus mastitis and metabolic immune pathways.[Pubmed:38568835]
FASEB J. 2024 Apr 15;38(7):e23587.
Mastitis is a disease characterized by congestion, swelling, and inflammation of the mammary gland and usually caused by infection with pathogenic microorganisms. Furthermore, the development of mastitis is closely linked to the exogenous pathway of the gastrointestinal tract. However, the regulatory mechanisms governing the gut-metabolism-mammary axis remain incompletely understood. The present study revealed alterations in the gut microbiota of mastitis rats characterized by an increased abundance of the Proteobacteria phylum. Plasma analysis revealed significantly higher levels of L-isoleucine and cholic acid along with 7-Ketodeoxycholic acid. Mammary tissue showed elevated levels of arachidonic acid metabolites and norlithocholic acid. Proteomic analysis showed increased levels of IFIH1, Tnfaip8l2, IRGM, and IRF5 in mastitis rats, which suggests that mastitis triggers an inflammatory response and immune stress. Follistatin (Fst) and progesterone receptor (Pgr) were significantly downregulated, raising the risk of breast cancer. Extracellular matrix (ECM) receptors and focal adhesion signaling pathways were downregulated, while blood-milk barrier integrity was disrupted. Analysis of protein-metabolic network regulation revealed that necroptosis, protein digestion and absorption, and arachidonic acid metabolism were the principal regulatory pathways involved in the development of mastitis. In short, the onset of mastitis leads to changes in the microbiota and alterations in the metabolic profiles of various biological samples, including colonic contents, plasma, and mammary tissue. Key manifestations include disturbances in bile acid metabolism, amino acid metabolism, and arachidonic acid metabolism. At the same time, the integrity of the blood-milk barrier is compromised while inflammation is promoted, thereby reducing cell adhesion in the mammary glands. These findings contribute to a more comprehensive understanding of the metabolic status of mastitis and provide new insights into its impact on the immune system.
Multi-omics analysis of fecal samples in colorectal cancer Egyptians patients: a pilot study.[Pubmed:37644393]
BMC Microbiol. 2023 Aug 29;23(1):238.
BACKGROUND: Colorectal cancer (CRC) is a public health concern and the second most common disease worldwide. This is due to genetic coding and is influenced by environmental aspects, in which the gut microbiota plays a significant role. The purpose of this study was to compare the microbiota makeup of CRC patients with that of healthy control and to identify upregulated and downregulated proteins and metabolites in CRC patients. Using a next-generation sequencing approach, fecal samples of five females (4 CRC patients and one healthy control) were analyzed by BGI DNBSEQ-T7, Hong Kong, China. Furthermore, proteomics and metabolomics analysis were performed using LC-MS/MS technique. RESULTS: Dysbiosis of gut microbiota has been observed in patients with CRC, with an increase in microbiota diversity at all taxonomic levels relative to healthy control. Where, at the functional level the bacterial species participate in many different pathways among them de novo nucleotide synthesis and amino acids pathways were aberrantly upregulated in CRC patients. Proteomics and metabolomics profiles of CRC patients showed different proteins and metabolites, a total of 360 and 158 proteins and metabolites, respectively were highly expressed compared to healthy control with fold change >/= 1.2. Among the highly expressed proteins were transketolase, sushi domain-containing protein, sulfide quinone oxidoreductase protein, AAA family ATPase protein, carbonic anhydrase, IgG Fc-binding protein, nucleoside diphosphate kinase protein, arylsulfatase, alkaline phosphatase protein, phosphoglycerate kinase, protein kinase domain-containing protein, non-specific serine/threonine protein kinase, Acyl-CoA synthetase and EF-hand domain-containing protein. Some of the differential metabolites, Taurine, Taurocholic acid, 7-Ketodeoxycholic acid, Glycochenodeoxycholic acid, Glycocholic acid, and Taurochenodeoxycholic acid that belong to bile acids metabolites. CONCLUSIONS: Some bacterial species, proteins, and metabolites could be used as diagnostic biomarkers for CRC. Our study paves an insight into using multi-omics technology to address the relationship between gut microbiota and CRC.
Dihydromyricetin ameliorated nonalcoholic steatohepatitis in mice by regulating the composition of serous lipids, bile acids and ileal microflora.[Pubmed:37533083]
Lipids Health Dis. 2023 Aug 2;22(1):112.
BACKGROUND: Dihydromyricetin (DMY) is a natural flavonoid with anti-nonalcoholic steatohepatitis (NASH) activity. However, the effects of DMY on the composition of lipids and bile acids (BAs) in serum, and gut microbiota (GM) in ileum of mice with NASH are not clear. METHODS: After male C57BL/6 mice was fed with methionine and choline deficiency (MCD) diet and simultaneously administered with DMY (300 mg/kg/day) by gavage for 8 weeks, the pathological changes of liver tissue were observed by Oil Red O, hematoxylin eosin and Masson staining, the levels of serum alaninea minotransferase, aspartate aminotransferase and liver triglyceride, malonic dialdehyde were detected by the detection kits, the composition and contents of serum lipids and BAs were detected by Liquid Chromatograph-Mass Spectrometry, the mRNA levels of hepatic BAs homeostasis-related genes were detected by RT-qPCR, and microbiological diversity in ileum was analyzed by 16S rDNA sequencing. RESULTS: The results showed that the significant changes including 29 lipids, 4 BAs (23-nor-deoxycholic acid, ursodeoxycholic acid, 7-Ketodeoxycholic acid and cholic acid), 2 BA transporters (Mrp2 and Oatp1b2) and 8 GMs between MCD and DMY groups. Among them, DMY treatment significantly down-regulated 21 lipids, 4 BAs mentioned above, the ratio of Firmicutes/Bacteroidota and the abundance of Erysipelotrichaceae, Faecalibacuium, significantly up-regulated 8 lipids and 5 GMs (Verrucomicrobiota, Bacteroidota, Actinobacteria, Akkermansiaceae and Akkermansia). CONCLUSIONS: The results suggested that DMY may alleviate MCD diet-induced NASH through decreasing the serum levels of toxic BAs which regulated by liver Oatp1b2 and Mrp2, regulating the metabolism of related lipids, and up-regulating intestinal probiotics (Actinobacteria and Verrucomicrobiota at the phylum level; Akkermansiaceae at the family level; Akkermansiaat at the genus level) and inhibiting intestinal harmful bacteria (Firmicutes at the phylum level; Erysipelotrichaceae at the family level; Faecalibaculum at the genus level).
Metabolomics Analysis Reveals Altered Metabolic Pathways and Response to Doxorubicin in Drug-Resistant Triple-Negative Breast Cancer Cells.[Pubmed:37512572]
Metabolites. 2023 Jul 20;13(7):865.
This study aimed to investigate metabolic changes following the acquisition of resistance to doxorubicin in the triple-negative breast cancer (TNBC) cell line MDA-MB-231. Two drug-resistant cell lines, DOX-RES-50 and DOX-RES-100, were generated by treating MDA-MB-231 cells with doxorubicin for 24 h and allowing them to recover for six weeks. Both drug-resistant cell lines demonstrated an increase in doxorubicin IC(50) values, indicating acquired drug resistance. Metabolomics analysis showed clear separation between the parental MDA-MB-231 cell line and the drug-resistant cell lines. Pathway analysis revealed that arginine and proline metabolism, glutathione metabolism, and beta-alanine metabolism were significantly perturbed in the drug-resistant cell lines compared to the parental cell line. After matching signals to an in-house library of reference standards, significant decreases in short- and medium-chain acylcarnitines and significant increases in long-chain acylcarnitines, 5-oxoproline, and 7-Ketodeoxycholic acid were observed in the resistant cell lines as compared to the parental MDA-MB-231 cell line. In addition to baseline metabolic differences, we also investigated differences in metabolic responses in resistant cell lines upon a second exposure at multiple concentrations. Results indicate that whereas the parental MDA-MB-231 cell line had many metabolites that responded to doxorubicin in a dose-dependent manner, the two resistant cell lines lost a dose-dependent response for the majority of these metabolites. The study's findings provide insight into how metabolism is altered during the acquisition of resistance in TNBC cells and how the metabolic response to doxorubicin changes upon repeated treatment. This information can potentially identify novel targets to prevent or reverse multi-drug resistance in TNBC, and also demonstrate the usefulness of metabolomics technology in identifying new mechanisms of drug resistance in cancer and potential drug targets.
The potential of Klebsiella and Escherichia-Shigella and amino acids metabolism to monitor patients with postmenopausal osteoporosis in northwest China.[Pubmed:37495941]
BMC Microbiol. 2023 Jul 26;23(1):199.
BACKGROUND: Intestinal flora has been proposed to mediate the occurrence of postmenopausal osteoporosis (PMO). However, the mechanism by which microbes and their metabolites interactively promote PMO remains unknown. METHODS: This study aimed to investigate changes in the intestinal flora and associated metabolites, and their role in PMO. 16S rRNA gene sequencing and metabolomics were performed to obtain postmenopausal women with osteopenia (lower bone mass, LBM), postmenopausal women with osteoporosis (OST), and healthy women as the control group. RESULTS: We identified taxa-specific and metabolite differences in the intestinal flora of the participants of this study. The pathogenic bacteria Klebsiella (0.59% and 0.71%, respectively) and Escherichia-Shigella (2.72% and 4.30%, respectively) were enriched in the LBM and OST groups (p < 0.05). Some short-chain fatty acid (SCFAs) producing bacteria, Lactobacillus, Akkermansia, Prevotella, Alistipes, and Butyricicoccus, were reduced in patients with LBM and OST compared to the control. Moreover, fecal metabolomic analyses suggested that the metabolites of indole-3-acetic acid and 7-Ketodeoxycholic acid were altered in the LBM and OST groups compared to the control (p < 0.05). Enrichment analysis suggested that valine, leucine, and isoleucine biosynthesis; aromatic amino acid biosynthesis; and phenylalanine metabolism were significantly associated with the identified microbiota biomarkers and OST. Moreover, metabolite marker signatures distinguished patients in the OST from those in the control group with an area under the curve (AUC) of 0.978 and 1.00 in the negative and positive ion modes, respectively. Finally, we also found that the fecal level of interleukin-10 (IL-10) in the OST group was significantly lower than that in the control group and LBM group (p < 0.05), while tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) were significantly higher in the OST group than that in the control group (p < 0.05). CONCLUSIONS: This study provides robust evidence connecting the intestinal flora and fecal metabolomics with PMO. Integrated metabolite and microbiota analyses demonstrated that in addition to dysregulated bacteria, indole-3-acetic acid, 7-Ketodeoxycholic acid, and other metabolites can be used for the distinguish of LBM and PMO.
Altered gut microbiota-host bile acid metabolism in IBS-D patients with liver depression and spleen deficiency pattern.[Pubmed:37468912]
Chin Med. 2023 Jul 19;18(1):87.
BACKGROUND: Dysregulation of gut microbiota-host bile acid (BA) co-metabolism is a critical pathogenic factor of diarrhea-predominant irritable bowel syndrome (IBS-D). Traditional Chinese Medicine (TCM), instructed by pattern differentiation, is effective in treating IBS-D, in which liver depression and spleen deficiency (LDSD) is the most prevalent pattern. Still, it is unclear the linkage between the LDSD pattern and the BA metabolic phenotype. PURPOSE: This study aimed to uncover the biological basis of the LDSD pattern from the BA metabolic perspective. METHODS: Patients with IBS-D completed questionnaires regarding the irritable bowel severity scoring system (IBS-SSS), stool frequency, Stool Bristol scale, and Self-Rating Scales of mental health. Fasting blood and morning feces were collected to analyze the gut metagenome and BA-related indices/metabolites. RESULTS: IBS-D patients with LDSD had a higher incidence of BA overexcretion (41% vs. 23% non-LDSD) with significant elevations in fecal total BAs and serum BA precursor 7alpha-hydroxy-4-cholesten-3-one levels. Compared to controls or non-LDSD patients, LDSD patients had a featured fecal BA profile, with higher proportions of deoxycholic acid (DCA), 7-Ketodeoxycholic acid, and lithocholic acid. It is consistent with the BA-metabolizing genomic changes in the LDSD gut microbiota characterized by overabundances of 7-dehydroxylating bacteria and BA-inducible genes (baiCD/E/H). The score of bowel symptoms (stool frequency and abdominal pain) showing greater severity in the LDSD pattern were positively correlated with bai-expressing bacterial abundances and fecal DCA levels separately. CONCLUSION: We clarified a differed BA metabolic phenotype in IBS patients with LDSD, which closely correlates with the severity of bowel symptoms. It demonstrates that gut microbiota and host co-metabolism of BAs would provide crucial insight into the biology of the LDSD pattern and its internal relationship with IBS progression.
Qing-Kai-Ling oral liquid alleviated pneumonia via regulation of intestinal flora and metabolites in rats.[Pubmed:37362920]
Front Microbiol. 2023 Jun 9;14:1194401.
BACKGROUND: Qing-Kai-Ling (QKL) oral liquid, evolving from a classical Chinese formula known as An-Gong-Niu-Huang pills, is a well-established treatment for pneumonia with its mechanism remaining muddled. Studies have shown that the regulation of both intestinal flora and host-microbiota co-metabolism may contribute to preventing and treating pneumonia. The study aimed to investigate the potential mechanism by which QKL alleviates pneumonia from the perspective of 'microbiota-metabolites-host' interaction. METHODS: We evaluated the therapeutic effects of QKL on lipopolysaccharide (LPS)-induced pneumonia rats. To explore the protective mechanism of QKL treatment, a multi-omics analysis that included 16S rDNA sequencing for disclosing the key intestinal flora, the fecal metabolome to discover the differential metabolites, and whole transcriptome sequencing of lung tissue to obtain the differentially expressed genes was carried out. Then, a Spearman correlation was employed to investigate the association between the intestinal flora, the fecal metabolome and inflammation-related indices. RESULTS: The study demonstrated that pneumonia symptoms were significantly attenuated in QKL-treated rats, including decreased TNF-alpha, NO levels and increased SOD level. Furthermore, QKL was effective in alleviating pneumonia and provided protection equivalent to that of the positive drug dexamethasone. Compared with the Model group, QKL treatment significantly increased the richness and alphalpha diversity of intestinal flora, and restored multiple intestinal genera (e.g., Bifidobacterium, Ruminococcus_torques_group, Dorea, Mucispirillum, and Staphylococcus) that were correlated with inflammation-related indices. Interestingly, the intestinal flora demonstrated a strong correlation with several metabolites impacted by QKL. Furthermore, metabolome and transcriptome analyses showed that enrichment of several host-microbiota co-metabolites [arachidonic acid, 8,11,14-eicosatrienoic acid, LysoPC (20:0/0:0), LysoPA (18:0e/0:0), cholic acid, 7-Ketodeoxycholic acid and 12-ketodeoxycholic acid] levels and varying lung gene (Pla2g2a, Pla2g5, Alox12e, Cyp4a8, Ccl19, and Ccl21) expression were observed in the QKL group. Moreover, these metabolites and genes were involved in arachidonic acid metabolism and inflammation-related pathways. CONCLUSION: Our findings indicated that QKL could potentially modulate intestinal flora dysbiosis, improve host-microbiota co-metabolism dysregulation and regulate gene expression in the lungs, thereby mitigating LPS-induced pneumonia in rats. The study may provide new ideas for the clinical application and further development of QKL.
Untargeted metabolomics unravel serum metabolic alterations in smokers with hypertension.[Pubmed:36935758]
Front Physiol. 2023 Mar 2;14:1127294.
Background: Cigarette smoking is an important environmental risk factor for cardiovascular events of hypertension (HTN). Existing studies have provided evidence supporting altered gut microbiota by cigarette smoking, especially in hypertensive patients. Metabolic biomarkers play a central role in the functional potentials of the gut microbiome but are poorly characterized in hypertensive smokers. To explore whether serum metabolomics signatures and compositions of HTN patients were varied in smokers, and investigate their connecting relationship to gut microbiota, the serum metabolites were examined in untreated hypertensive patients using untargeted liquid chromatography-mass spectrometry (LC/MS) analysis. Results: A dramatic difference and clear separation in community features of circulating metabolomics members were seen in smoking HTN patients compared with the non-smoking controls, according to partial least squares discrimination analysis (PLS-DA) and orthogonal partial least squares discrimination analysis (OPLS-DA). Serum metabolic profiles and compositions of smoking patients with HTN were significantly distinct from the controls, and were characterized by enrichment of 12-HETE, 7-Ketodeoxycholic acid, Serotonin, N-Stearoyl tyrosine and Deoxycholic acid glycine conjugate, and the depletion of Tetradecanedioic acid, Hippuric acid, Glyceric acid, 20-Hydroxyeicosatetraenoic acid, Phenylpyruvic acid and Capric acid. Additionally, the metabolome displayed prominent functional signatures, with a majority proportion of the metabolites identified to be discriminating between groups distributed in Starch and sucrose metabolism, Caffeine metabolism, Pyruvate metabolism, Glycine, serine and threonine metabolism, and Phenylalanine metabolic pathways. Furthermore, the observation of alterations in metabolites associated with intestinal microbial taxonomy indicated that these metabolic members might mediate the effects of gut microbiome on the smoking host. Indeed, the metabolites specific to smoking HTNs were strongly organized into co-abundance networks, interacting with an array of clinical parameters, including uric acid (UA), low-denstiy lipoprotein cholesterol (LDLC) and smoking index. Conclusions: In conclusion, we demonstrated disparate circulating blood metabolome composition and functional potentials in hypertensive smokers, showing a linkage between specific metabolites in blood and the gut microbiome.
Chaihu-shugan-san alleviates depression-like behavior in mice exposed to chronic unpredictable stress by altering the gut microbiota and levels of the bile acids hyocholic acid and 7-ketoDCA.[Pubmed:36339629]
Front Pharmacol. 2022 Oct 19;13:1040591.
Chaihu-Shugan-San (CSS) is a traditional botanical drug formula often prescribed to treat depression in oriental countries, but its pharmacotherapeutic mechanism remains unknown. It was recently reported that CSS alters the composition of intestinal microflora and related metabolites such as bile acids (BAs). Since the intestinal microflora affects physiological functions of the brain through the gut-microbiota-brain axis, herein we investigated whether CSS altered BA levels, gut microflora, and depression-like symptoms in chronic unpredictable mild stress (CUMS) mice, a well-established mouse model of depression. Furthermore, we determined whether BA manipulation and fecal microbiota transplantation altered CSS antidepressant actions. We found that the BA chelator cholestyramine impaired the antidepressant effects of CSS, which was partially rescued by dietary cholic acid. CSS increased the relative abundance of Parabacteroides distasonis in the colon of CUMS mice, and increased serum levels of various BAs including hyocholic acid (HCA) and 7-Ketodeoxycholic acid (7-ketoDCA). Furthermore, gut bacteria transplantation from CSS-treated mice into untreated or cholestyramine-treated CUMS mice restored serum levels of HCA and 7-ketoDCA, alleviating depression-like symptoms. In the hippocampus, CSS-treated mice had decreased expression of genes associated with BA transport (Bsep and Fxr) and increased expression of brain-derived neurotrophic factor and its receptor, TrkB. Overall, CSS increases intestinal P. distasonis abundance, leading to elevated levels of secondary BAs in the circulation and altered expression of hippocampal genes implicated in BA transport and neurotrophic signaling. Our data strongly suggest that the gut microbiota-brain axis contributes to the potent antidepressant action of CSS by modulating BA metabolism.
A dynamic multiple reaction monitoring strategy to develop and optimize targeted metabolomics methods: Analyzing bile acids in capecitabine-induced diarrhea.[Pubmed:35850015]
J Pharm Biomed Anal. 2022 Sep 20;219:114938.
OBJECTIVE: We sought to develop and optimize a targeted bile acids (BAs) metabolomics method based on a dynamic multiple reaction monitoring (dMRM) strategy and explored the dynamic alterations of BAs in diarrhea induced by capecitabine in a mouse model. METHOD: The targeted metabolomics method was developed using an Agilent 6460A triple quadrupole mass spectrometer, and 41 types of BAs were monitored in negative ionization mode. The mass spectrometer detection was optimized using dMRM to enhance the responses, separation, and peak shape and to shorten the analysis time. A mouse model of diarrhea was established by multiple administration of capecitabine, and plasma samples were collected at baseline and the end of drug administration for subsequent BAs analysis. RESULTS: The targeted BA metabolomics method achieved shorter chromatographic separation time (10 min) for 41 BAs, with good peak shapes and response increases of 3- to 10-fold after application of dMRM. The mouse model of capecitabine-induced diarrhea was established, and the three BAs 23-norcholic acid, isolithocholic acid, and isodeoxycholic acid in the baseline samples contributed the most to differentiating mice with diarrhea from those without diarrhea. For mice that ultimately developed diarrhea, apocholic acid, isodeoxycholic acid, and 7-Ketodeoxycholic acid exhibited the largest change in concentrations compared with their baseline concentrations. CONCLUSION: The dMRM strategy has obvious advantages compared with common MRM. The results in model mice showed that a differentiated profile of BAs in the baseline may indicate biomarkers of diarrhea induced by capecitabine, and disturbed homeostasis may explain the metabolomic mechanism of diarrhea occurrence.
FuZhengHuaYuJiangZhuTongLuoFang Prescription Modulates Gut Microbiota and Gut-Derived Metabolites in UUO Rats.[Pubmed:35669118]
Front Cell Infect Microbiol. 2022 May 20;12:837205.
BACKGROUND: Alteration of intestinal flora and metabolites is closely related to chronic kidney disease (CKD) across early to advanced stages. FuZhengHuaYuJiangZhuTongLuoFang prescription (FZHY) is a Chinese herb that has been proven to effectively treat CKD, but the underlying mechanism is not clear. METHODS: Rats were subjected to intragastric treatment with FZHY 7, 14, and 21 days after unilateral ureteral obstruction (UUO) surgery, and kidney tissue, colon tissue, serum, and stool samples were collected. RESULTS: FZHY treatment effectively ameliorated UUO-induced renal function loss, renal injury and renal fibrosis, and colon tissue damage and fibrosis on day 7. The results of 16S flora analysis (day 7) showed that, compared with the UUO group, both the FZHY group and the sham group showed decreased levels of g_Monoglobus, g_Papillibacter, g_Eubacterium_nodatum, and g_Family_XIII_AD3011. Additionally, FZHY obviously induced the reduction of serum citrulline, glycoursodeoxycholic acid, 23-nordeoxycholic acid, 7-Ketodeoxycholic acid, kahweol, lipoid B4, 4-(3,4-dihydro-2H-1,5-benzodioxepin-7-yl)-2-methyl-1,3-thiazole, taurolithocholic acid sodium salt, indoline-2-carboxylic acid, 5(S),15(S)-diHETE, and others and the increase of bilirubin, asparagine, and others, which were positively associated with the above four candidate bacteria. Moreover, FZHY increased the levels of ZO-1, occludin, and claudin-1 in the colonic mucosa and reduced the levels of CRP, TNF-alpha, IL-6, and IL-1 in the serum and LN, FN, Col-I, and Col-III in the tubulointerstitium of UUO rats on day 7. CONCLUSION: Our study revealed that FZHY reduced kidney damage at the early stage of CKD by regulating the above four candidate bacteria biomarkers and gut-derived harmful metabolites, inhibiting the inflammation response and tubulointerstitial fibrosis, providing deep insight into CKD therapeutic strategy.
Metabolic reprogramming of the intestinal microbiome with functional bile acid changes underlie the development of NAFLD.[Pubmed:35561146]
Hepatology. 2022 Dec;76(6):1811-1824.
BACKGROUND AND AIMS: Bile acids are hepatic metabolites and have many properties considered to be relevant to the pathophysiology of NAFLD. Circulating levels of the intestinal microbiome-modified bile acid deoxycholate are increased in cirrhosis. APPROACH AND RESULTS: To further elucidate the role of bile acids and intestinal microbiota linked to bile acids in progressively severe NAFLD, a multiomic study of feces including 16S rRNA sequencing, microbial transcriptomics and metabolomics was performed in a cohort with varying phenotypes of NAFLD. Several bile acids of microbial origin derived from deoxycholic acid (DCA) (glycodeoxycholate, 7-Ketodeoxycholic acid, dehydrocholic acid) increased with disease activity and fibrosis stage. These were linked to increased expression of microbial bile salt hydrolase, bile acid operon (BaiCD) and hydroxysteroid dehydrogenases (hdhA) required for DCA and downstream metabolite synthesis providing a mechanistic basis for altered bile acid profiles with disease progression. Bacteroidetes and several genera of Lachnospiraceae family containing DCA generating genes increased with increasing disease severity, whereas several potentially beneficial microbes sensitive to antibacterial effects of DCA e.g., Ruminococcaceae were decreased. The clinical relevance of these data was confirmed in an independent cohort enrolled in a clinical trial for NASH where at entry DCA and its conjugates were associated with advanced fibrosis. In patients treated with placebo, DCA declined in those with fibrosis regression and increased in those with fibrosis progression. DCA rose further in those with compensated cirrhosis when they experienced decompensation. CONCLUSIONS: These findings demonstrate a role for bile acids and the bile acid dependent microbiome in the development and progression of NAFLD and set the stage to leverage these findings for NASH biomarker development and for therapeutics.
Crocetin Prolongs Recovery Period of DSS-Induced Colitis via Altering Intestinal Microbiome and Increasing Intestinal Permeability.[Pubmed:35409192]
Int J Mol Sci. 2022 Mar 30;23(7):3832.
Crocetin is one of the major active constituents of saffron (Crocus sativus L.) which has a reputation for facilitating blood circulation and dispersing blood stasis in traditional Chinese medicine. However, there is little evidence showing the relationship between crocetin intake and the risk of gastrointestinal diseases such as colitis. In order to investigate the effect of crocetin on the regulation of intestinal barrier function and intestinal microbiota composition, mice were treated with crocetin after 3% dextran sulfate sodium (DSS) administration for one week. We found that crocetin intake at 10 mg/kg aggravated colitis in mice, showing increased weight loss and more serious histological abnormalities compared with the DSS group. The 16s rDNA sequencing analysis of the feces samples showed that mice treated with 10 mg/kg crocetin had lower species diversity and richness than those treated with DSS. At the genus level, a higher abundance of Akkermansia and Mediterraneibacter, and a lower abundance of Muribaculaceae, Dubosiella, Paramuribaculum, Parasutterella, Allobaculum, Duncaniella, Candidatus Stoquefichus, and Coriobacteriaceae UCG-002 were observed in the crocetin group. Untargeted metabolomic analyses revealed that crocetin reduced the levels of primary and secondary bile acids such as 12-ketodeoxycholic acid, 7-Ketodeoxycholic acid, 3-sulfodeoxycholic acid, 6-ethylchenodeoxycholic acid, chenodeoxycholate, glycochenodeoxycholate-7-sulfate, glycocholate, and sulfolithocholic acid in the colon. In conclusion, crocetin intake disturbed intestinal homeostasis and prolonged recovery of colitis by promoting inflammation and altering gut microbiota composition and its metabolic products in mice. Our findings suggest that patients with gastrointestinal diseases such as inflammatory bowel disease should use crocetin with caution.
Metabolites of gut microbiota fermenting Poria cocos polysaccharide alleviates chronic nonbacterial prostatitis in rats.[Pubmed:35398386]
Int J Biol Macromol. 2022 Jun 1;209(Pt B):1593-1604.
Chronic nonbacterial prostatitis (CNP) is a common urology disease. Our previous research found Poria cocos polysaccharides (PPs) alleviated CNP and suggested the effect was related to gut bacteria. We investigated the crucial bacteria and their metabolites responsible for the anti-CNP effect to discover possible mechanisms. The results showed that after the fermentation of PPs by human fecal microbiota, Parabacteroides, Fusicatenibacter, and Parasutterella were significantly enriched. Haloperidol glucuronide and 7-Ketodeoxycholic acid generated by these bacteria could be responsible for the increased expression of Alox15 and Pla2g2f and the reduced expression of Cyp1a1 and Hsd17b7 in colon epithelium. The ratio of dihydrotestosterone to estradiol in serum was regulated, and CNP was alleviated. Our results suggested that Parabacteroides, Fusicatenibacter, and Parasutterella could be the essential bacteria in CNP alleviation and their metabolites of PPs 7-Ketodeoxycholic acid and haloperidol glucuronide could be the signal molecules of the "gut-prostate axis".


