SalsolinolCAS# 27740-96-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 27740-96-1 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
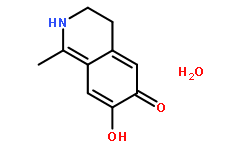
| Formula | C10H11NO2.H2O | M.Wt | 195.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Salsolinol Dilution Calculator

Salsolinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1224 mL | 25.6121 mL | 51.2243 mL | 102.4485 mL | 128.0606 mL |
| 5 mM | 1.0245 mL | 5.1224 mL | 10.2449 mL | 20.4897 mL | 25.6121 mL |
| 10 mM | 0.5122 mL | 2.5612 mL | 5.1224 mL | 10.2449 mL | 12.8061 mL |
| 50 mM | 0.1024 mL | 0.5122 mL | 1.0245 mL | 2.049 mL | 2.5612 mL |
| 100 mM | 0.0512 mL | 0.2561 mL | 0.5122 mL | 1.0245 mL | 1.2806 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bufotenidine
Catalog No.:BCX0714
CAS No.:487-91-2
- Epigallocatechin gallate octaacetate
Catalog No.:BCX0713
CAS No.:148707-39-5
- Oleandrin,anhydro-16-deacetyl-
Catalog No.:BCX0712
CAS No.:69549-58-2
- Pinolenic acid
Catalog No.:BCX0711
CAS No.:16833-54-8
- Digitoxigenin
Catalog No.:BCX0710
CAS No.:143-62-4
- Oleandrigenin
Catalog No.:BCX0709
CAS No.:465-15-6
- Oroxylin A-7-glucoside
Catalog No.:BCX0708
CAS No.:36948-77-3
- 5-Hydroxy-2′,3,4′,7-tetramethoxyflavone
Catalog No.:BCX0707
CAS No.:19056-75-8
- Methyl jasmonate
Catalog No.:BCX0706
CAS No.:1211-29-6
- Ginsenoside Ra6
Catalog No.:BCX0705
CAS No.:1346522-89-1
- Endothalic acid
Catalog No.:BCX0704
CAS No.:145-73-3
- Epirosmanol
Catalog No.:BCX0703
CAS No.:93380-12-2
- Prunetrin
Catalog No.:BCX0716
CAS No.:154-36-9
- limocitrin -3-O-rutinoside
Catalog No.:BCX0717
CAS No.:79384-27-3
- Anthrone
Catalog No.:BCX0718
CAS No.:90-44-8
- D-Lyxose
Catalog No.:BCX0719
CAS No.:1114-34-7
- Ligupurpuroside D
Catalog No.:BCX0720
CAS No.:1194056-35-3
- 7-Ketodeoxycholic acid
Catalog No.:BCX0721
CAS No.:911-40-0
- Rabdosiin
Catalog No.:BCX0722
CAS No.:119152-54-4
- Melibiose
Catalog No.:BCX0723
CAS No.:585-99-9
- Neoanhydropodophyllol
Catalog No.:BCX0724
CAS No.:62287-47-2
- (+)-Rabdosiin
Catalog No.:BCX0725
CAS No.:263397-69-9
- 6-Methoxy-2-(2-phenylethyl)chromone
Catalog No.:BCX0726
CAS No.:84294-89-3
- 6-Methoxy-2-[2-(3'-methoxyphenyl)ethyl]chromone
Catalog No.:BCX0727
CAS No.:84294-88-2
[Differences in chemical components in processing of dried ginger-steamed, sand-fried, and rice swill water-bleached Aconiti Lateralis Radix Praeparata pieces in "Jianchang" faction based on UPLC-MS/MS].[Pubmed:38211995]
Zhongguo Zhong Yao Za Zhi. 2023 Dec;48(23):6387-6395.
This study compared the changes in chemical components during the processing of different types of Aconiti Lateralis Radix Praeparata(ALRP) in "Jianchang" faction, i.e., dried ginger-steamed ALRP pieces(Yin-FP), sand-fried ALRP pieces(Yang-FP), and rice swill water-bleached ALRP pieces(DFP), and provided a scientific basis for the mechanism in toxicity reduction and efficacy enhancement from a compositional perspective. Samples were collected during the processing of the three types of ALRP pieces, yielding raw ALRP pieces, water-bleached Yin-FP, ginger juice-moistened Yin-FP, steamed Yin-FP, water-bleached Yang-FP, sand-fried Yang-FP, water-bleached DFP, rice swill water-bleached DFP, and roasted DFP. Aconitine, mesaconitine, hypaconitine, benzoylaconine, benzoylmesaconine, benzoylhypaconine, aconine, mesaconine, hypaconine, Salsolinol, fuziline, and higenamine in the extracts were determined by UPLC-MS/MS, and then content analysis and cluster heatmap analysis were performed on 11 sets of samples. During the processing of the three types of ALRP pieces, bleaching significantly reduced the content of 12 alkaloids; steaming, stir-frying, and roasting significantly reduced the content of diester-type alkaloids(aconitine, mesaconitine, and hypaconitine) and significantly increased the content of monoester-type alkaloids(benzoylaconine, benzoylmesaconine, and benzoylhypaconine) and aminoalcohol-type alkaloids(aconine, mesaconine, and hypaconine). During the processing of Yin-FP, the diester-type alkaloids continuously decreased, while the monoester-type and aminoalcohol-type alkaloids showed an initial decrease followed by an increase. During the processing of Yin-FP, Yang-FP, and DFP, the diester-type alkaloids continuously decreased, while the monoester-type and aminoalcohol-type alkaloids showed an initial decrease followed by an increase. Steamed Yin-FP showed a higher increase in content than fried Yang-FP and roasted DFP. Comprehensive analysis of content differences in toxic and therapeutic components in three ALRP pieces suggests that the distinctive processing methods in "Jianchang" faction can indeed achieve detoxification and efficacy enhancement on ALRP. This study provides references for understanding the mechanisms of action of the three processing methods.
Butyrate Protects and Synergizes with Nicotine against Iron- and Manganese-induced Toxicities in Cell Culture.[Pubmed:38095760]
Neurotox Res. 2023 Dec 14;42(1):3.
Toxic exposures to heavy metals, such as iron (Fe) and manganese (Mn), can result in long-range neurological diseases and are therefore of significant environmental and medical concerns. We have previously reported that damage to neuroblastoma-derived dopaminergic cells (SH-SY5Y) by both Fe and Mn could be prevented by pre-treatment with nicotine. Moreover, butyrate, a short chain fatty acid (SCFA) provided protection against Salsolinol, a selective dopaminergic toxin, in the same cell line. Here, we broadened the investigation to determine whether butyrate might also protect against Fe and/or Mn, and whether, if combined with nicotine, an additive or synergistic effect might be observed. Both butyrate and nicotine concentration-dependently blocked Fe and Mn toxicities. Ineffective concentrations of nicotine and butyrate, when combined, provided full protection against both Fe and Mn. Moreover, the effects of nicotine but not butyrate could be blocked by mecamylamine, a non-selective nicotinic antagonist. On the other hand, the effects of butyrate, but not nicotine, could be blocked by beta-hydroxy butyrate, a fatty acid-3 receptor antagonist. These results not only provide further support for neuroprotective effects of both nicotine and butyrate but also indicate distinct mechanisms of action for each one. Furthermore, potential utility of butyrate and nicotine combination against heavy metal toxicities is suggested.
Butyrate protects and synergizes with nicotine against iron- and manganese-induced toxicities in cell culture: Implications for neurodegenerative diseases.[Pubmed:37886507]
Res Sq [Preprint]. 2023 Oct 5:rs.3.rs-3389904.
Toxic exposures to heavy metals, such as iron (Fe) and manganese (Mn), can result in long-range neurological diseases and are therefore of significant environmental and medical concerns. We have previously reported that damage to neuroblastoma-derived dopaminergic cells (SH-SY5Y) by both Fe and Mn could be prevented by pre-treatment with nicotine. Moreover, butyrate, a short chain fatty acid (SCFA) provided protection against Salsolinol, a selective dopaminergic toxin, in the same cell line. Here, we broadened the investigation to determine whether butyrate might also protect against Fe and/or Mn, and whether, if combined with nicotine, an additive or synergistic effect might be observed. Both butyrate and nicotine concentration-dependently blocked Fe and Mn toxicities. The ineffective concentrations of nicotine and butyrate, when combined, provided full protection against both Fe and Mn. Moreover, the effects of nicotine but not butyrate could be blocked by mecamylamine, a non-selective nicotinic antagonist. On the other hand, the effects of butyrate, but not nicotine, could be blocked by beta-hydroxy butyrate, a fatty acid-3 receptor antagonist. These results not only provide further support for neuroprotective effects of both nicotine and butyrate but indicate distinct mechanisms of action for each one. Furthermore, potential utility of the combination of butyrate and nicotine against heavy metal toxicities is suggested.
Salsolinol improves angiotensin II‑induced myocardial fibrosis in vitro via inhibition of LSD1 through regulation of the STAT3/Notch‑1 signaling pathway.[Pubmed:37869646]
Exp Ther Med. 2023 Sep 27;26(5):527.
The clinical incidence of congestive heart failure (CHF) is very high and it poses a significant threat to the health of patients. The traditional Chinese medicine monomer Salsolinol is widely used to treat similar symptoms of CHF. However, there have been no reports on the effect of Salsolinol for the management of CHF and its effects on myocardial fibrosis. In the present study, Salsolinol was used to treat angiotensin II (AngII)-induced human cardiac fibroblasts (HCFs) and cell proliferation and migration were assessed using a CCK-8, EdU staining assay and wound healing assay. Subsequently, immunofluorescence, western blotting and other techniques were used to detect indicators associated with cell fibrosis and relevant kits were used to detect markers of cellular inflammation and reactive oxygen species (ROS) production. Molecular docking analysis was used to predict the relationship between Salsolinol and lysine-specific histone demethylase 1A (LSD1). Subsequently, the expression of LSD1 in the serum of CHF patients was detected by reverse transcription-quantitative PCR. Finally, LSD1 was overexpressed in cells to explore the regulatory mechanism of Salsolinol in AngII-induced HFCs. Salsolinol reduced the proliferation and migration. Salsolinol reduced the expression of fibrosis marker proteins alpha-smooth muscle actin, Collagen I and Collagen III in a concentration-dependent manner, thereby reducing cell fibrosis. In addition, Salsolinol reduced the levels of TNF-alpha and IL-6 in the cell supernatant and ROS production following AngII induction. Salsolinol inhibited LSD1 expression and regulated the STAT3/Notch-1 signaling pathway. Upregulation of LSD1 reversed the effects of Salsolinol on AngII-induced HCFs. Salsolinol inhibited LSD1 via regulation of the STAT3/Notch-1 signaling pathway to improve Ang II-induced myocardial fibrosis in vitro.
Neuroprotective Activity of Enantiomers of Salsolinol and N-Methyl-(R)-salsolinol: In Vitro and In Silico Studies.[Pubmed:37867702]
ACS Omega. 2023 Oct 5;8(41):38566-38576.
Salsolinol (1-methyl-1,2,3,4-tetrahydroisoquinoline-6,7-diol) is a close structural analogue of dopamine with an asymmetric center at the C1 position, and its presence in vivo, both in humans and rodents, has already been proven. Yet, given the fact that Salsolinol colocalizes with dopamine-rich regions and was first detected in the urine of Parkinson's disease patients, its direct role in the process of neurodegeneration has been proposed. Here, we report that R and S enantiomers of Salsolinol, which we purified from commercially available racemic mixture by means of high-performance liquid chromatography, exhibited neuroprotective properties (at the concentration of 50 muM) toward the human dopaminergic SH-SY5Y neuroblastoma cell line. Furthermore, within the study, we observed no toxic effect of N-methyl-(R)-Salsolinol on SH-SY5Y neuroblastoma cells up to the concentration of 750 muM, either. Additionally, our molecular docking analysis showed that enantiomers of Salsolinol should exhibit a distinct ability to interact with dopamine D2 receptors. Thus, we postulate that our results highlight the need to acknowledge Salsolinol as an active dopamine metabolite and to further explore the neuroregulatory role of enantiomers of Salsolinol.
Metabonomics Analysis of Brain Stem Tissue in Rats with Primary Brain Stem Injury Caused Death.[Pubmed:37859476]
Fa Yi Xue Za Zhi. 2023 Aug 25;39(4):373-381.
OBJECTIVES: To explore the potential biomarkers for the diagnosis of primary brain stem injury (PBSI) by using metabonomics method to observe the changes of metabolites in rats with PBSI caused death. METHODS: PBSI, non-brain stem brain injury and decapitation rat models were established, and metabolic maps of brain stem were obtained by LC-MS metabonomics method and annotated to the HMDB database. Partial least square-discriminant analysis (PLS-DA) and random forest methods were used to screen potential biomarkers associated with PBSI diagnosis. RESULTS: Eighty-six potential metabolic markers associated with PBSI were screened by PLS-DA. They were modeled and predicted by random forest algorithm with an accuracy rate of 83.3%. The 818 metabolic markers annotated to HMDB database were used for random forest modeling and prediction, and the accuracy rate was 88.9%. According to the importance in the identification of cause of death, the most important metabolic markers that were significantly up-regulated in PBSI group were HMDB0038126 (genipinic acid, GA), HMDB0013272 (N-lauroylglycine), HMDB0005199 [(R)-Salsolinol] and HMDB0013645 (N,N-dimethylsphingosine). CONCLUSIONS: GA, N-lauroylglycine, (R)-Salsolinol and N,N-dimethylsphingosine are expected to be important metabolite indicators in the diagnosis of PBSI caused death, thus providing clues for forensic medicine practice.
Synthesis and neuroprotective activity of 3-aryl-3-azetidinyl acetic acid methyl ester derivatives.[Pubmed:37797174]
Arch Pharm (Weinheim). 2023 Dec;356(12):e2300378.
A library of 3-aryl-3-azetidinyl acetic acid methyl ester derivatives was prepared from N-Boc-3-azetidinone employing the Horner-Wadsworth-Emmons reaction, rhodium(I)-catalyzed conjugate addition of arylboronic acids, and subsequent elaborations to obtain N-unprotected hydrochlorides, N-alkylated and N-acylated azetidine derivatives. The compounds were evaluated for acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity, revealing several derivatives to possess AChE inhibition comparable to that of the AChE inhibitor rivastigmine. The binding mode of the AChE inhibitor donepezil and selected active compounds 26 and 27 within the active site of AChE was studied using molecular docking. Furthermore, the neuroprotective activity of the prepared compounds was evaluated in models associated with Parkinson's disease (Salsolinol-induced) and aspects of Alzheimer's disease (glutamate-induced oxidative damage). Compound 28 showed the highest neuroprotective effect in both Salsolinol- and glutamate-induced neurodegeneration models, and its protective effect in the glutamate model was revealed to be driven by a reduction in oxidative stress and caspase-3/7 activity.
Anti-inflammatory effects of banana (Musa balbisiana) peel extract on acne vulgaris: In vivo and in silico study.[Pubmed:37693819]
J Taibah Univ Med Sci. 2023 Jul 29;18(6):1586-1598.
OBJECTIVE: Acne vulgaris (AV) is a common problem with a relatively high incidence rate among Asian people. The potential antimicrobial and anti-inflammatory properties of banana peels have been demonstrated in previous studies but have not been studied in cases of AV. Therefore, this study was aimed at investigating the protective effects of banana (Musa balbisiana) peel extract (MBPE) against AV. METHODS: Thirty rats were divided into five groups (n = 6 rats per group): an AV group, AV group treated with 0.15% MBPE, AV group administered 0.30% MBPE, AV group administered 0.60% MBPE, and AV group administered clindamycin (the standard drug treatment). We assessed nodule size, bacterial count, histopathology, and cytokine levels (IL-1alpha, IFN-gamma, tumor necrosis factor (TNF)-alpha, and IL-8). Enzyme linked immunoassays were used to measure the cytokine levels. In addition, we performed molecular docking studies to determine the interactions between phytochemicals (trigonelline, vanillin, ferulic acid, isovanillic acid, rutin, and Salsolinol) via the Toll-like receptor 2 (TLR2) and nuclear factor-kappa B (NF-kappaB) pathways. RESULTS: All MBPE treatment groups, compared with the AV group, showed suppression of both bacterial growth and proinflammatory cytokine production, as well as resolved tissue inflammation. The nodule size was significantly suppressed in the groups receiving the two highest doses of MBPE, compared with the AV group. However, the pharmacological action of MBPE remained inferior to that of clindamycin. Docking studies demonstrated that rutin was the phytocompound with the most negative interaction energy with TLR2 or NF-kappaB. CONCLUSIONS: Our results indicated that MBPE has anti-inflammatory effects against AV, by suppressing nodule formation, inhibiting bacterial growth, and decreasing proinflammatory cytokine production.
The rostromedial tegmental nucleus RMTg is not a critical site for ethanol-induced motor activation in rats.[Pubmed:37474756]
Psychopharmacology (Berl). 2023 Oct;240(10):2071-2080.
RATIONALE: Opioid drugs indirectly activate dopamine (DA) neurons in the ventral tegmental area (VTA) through a disinhibition mechanism mediated by mu opioid receptors (MORs) present both on the GABA projection neurons located in the medial tegmental nucleus/tail of the VTA (RMTg/tVTA) and on the VTA GABA interneurons. It is well demonstrated that ethanol, like opioid drugs, provokes VTA DA neuron disinhibition by interacting (through its secondary metabolite, Salsolinol) with MORs present in VTA GABA interneurons, but it is not known whether ethanol could disinhibit VTA DA neurons through the MORs present in the RMTg/tVTA. OBJECTIVES: The objective of the present study was to determine whether ethanol, directly microinjected into the tVTA/RMTg, is also able to induce VTA DA neurons disinhibition. METHODS: Disinhibition of VTA DA neurons was indirectly assessed through the analysis of the motor activity of rats. Cannulae were placed into the tVTA/RMTg to perform microinjections of DAMGO (0.13 nmol), ethanol (150 or 300 nmol) or acetaldehyde (250 nmol) in animals pre-treated with either aCSF or the irreversible antagonist of MORs, beta-funaltrexamine (beta-FNA; 2.5 nmol). After injections, spontaneous activity was monitored for 30 min. RESULTS: Neither ethanol nor acetaldehyde directly administered into the RMTg/tVTA were able to increase the locomotor activity of rats at doses that, in previous studies performed in the posterior VTA, were effective in increasing motor activities. However, microinjections of 0.13 nmol of DAMGO into the tVTA/RMTg significantly increased the locomotor activity of rats. These activating effects were reduced by local pre-treatment of rats with beta-FNA (2.5 nmol). CONCLUSIONS: The tVTA/RMTg does not appear to be a key brain region for the disinhibiting action of ethanol on VTA DA neurons. The absence of dopamine in the tVTA/RMTg extracellular medium, the lack of local ethanol metabolism or both could explain the present results.
Temperature-regulated metabolites of Serratiamarcescens inhibited reproduction of pinewood nematode Bursaphelenchus xylophilus.[Pubmed:37416473]
iScience. 2023 Jun 8;26(7):107082.
The pinewood nematode Bursaphelenchus xylophilus is an invasive and destructive pathogen in forestry. Serratia marcescens AHPC29 was previously found to have nematicidal activity on B. xylophilus. The effect of AHPC29 growth temperature on B. xylophilus inhibition is unknown. Here we show that AHPC29 cultured at 15 degrees C or 25 degrees C, but not 37 degrees C, inhibited B. xylophilus reproduction. Metabolomic analysis found 31 up-regulated metabolites as potential effective substances in this temperature-related difference, with five of them were tested to be effective in inhibiting B. xylophilus reproduction. Among the five metabolites, Salsolinol was further verified in bacterial cultures with effective inhibition concentrations. This study found the inhibition of S. marcescens AHPC29 on B. xylophilus reproduction was temperature regulated and the differently expressed metabolites Salsolinol played roles in this temperature-regulated effect, which implies the capability of S. marcescens and its metabolites as promising new agents for the management of B. xylophilus.
Role of Metabolism on Alcohol Preference, Addiction, and Treatment.[Pubmed:37221350]
Curr Top Behav Neurosci. 2023 May 24.
Studies presented in this chapter show that: (1) in the brain, ethanol is metabolized by catalase to acetaldehyde, which condenses with dopamine forming Salsolinol; (2) acetaldehyde-derived Salsolinol increases the release of dopamine mediating, via opioid receptors, the reinforcing effects of ethanol during the acquisition of ethanol consumption, while (3) brain acetaldehyde does not influence the maintenance of chronic ethanol intake, it is suggested that a learned cue-induced hyperglutamatergic system takes precedence over the dopaminergic system. However, (4) following a prolonged ethanol deprivation, the generation of acetaldehyde in the brain again plays a role, contributing to the increase in ethanol intake observed during ethanol re-access, called the alcohol deprivation effect (ADE), a model of relapse behavior; (5) naltrexone inhibits the high ethanol intake seen in the ADE condition, suggesting that acetaldehyde-derived Salsolinol via opioid receptors also contributes to the relapse-like drinking behavior. The reader is referred to glutamate-mediated mechanisms that trigger the cue-associated alcohol-seeking and that also contribute to triggering relapse.
Dietary Sources of Anthocyanins and Their Association with Metabolome Biomarkers and Cardiometabolic Risk Factors in an Observational Study.[Pubmed:36904207]
Nutrients. 2023 Feb 28;15(5):1208.
Anthocyanins (ACNs) are (poly)phenols associated with reduced cardiometabolic risk. Associations between dietary intake, microbial metabolism, and cardiometabolic health benefits of ACNs have not been fully characterized. Our aims were to study the association between ACN intake, considering its dietary sources, and plasma metabolites, and to relate them with cardiometabolic risk factors in an observational study. A total of 1351 samples from 624 participants (55% female, mean age: 45 +/- 12 years old) enrolled in the DCH-NG MAX study were studied using a targeted metabolomic analysis. Twenty-four-hour dietary recalls were used to collect dietary data at baseline, six, and twelve months. ACN content of foods was calculated using Phenol Explorer and foods were categorized into food groups. The median intake of total ACNs was 1.6mg/day. Using mixed graphical models, ACNs from different foods showed specific associations with plasma metabolome biomarkers. Combining these results with censored regression analysis, metabolites associated with ACNs intake were: Salsolinol sulfate, 4-methylcatechol sulfate, linoleoyl carnitine, 3,4-dihydroxyphenylacetic acid, and one valerolactone. Salsolinol sulfate and 4-methylcatechol sulfate, both related to the intake of ACNs mainly from berries, were inversely associated with visceral adipose tissue. In conclusion, plasma metabolome biomarkers of dietary ACNs depended on the dietary source and some of them, such as Salsolinol sulfate and 4-methylcatechol sulfate may link berry intake with cardiometabolic health benefits.
Dihydromyricetin Protects Against Salsolinol-Induced Toxicity in Dopaminergic Cell Line: Implication for Parkinson's Disease.[Pubmed:36585544]
Neurotox Res. 2023 Apr;41(2):141-148.
Parkinson's disease (PD) is a progressive neurodegenerative disease associated with loss of dopaminergic neurons in the substantia nigra pars compacta. Although aging is the primary cause, environmental and genetic factors have also been implicated in its etiology. In fact, the sporadic nature of PD (i.e., unknown etiology) renders the uncovering of the exact pathogenic mechanism(s) or development of effective pharmacotherapies challenging. In search of novel neuroprotectants, we showed that butyrate (BUT), a short-chain fatty acid, protects against Salsolinol (SALS)-induced toxicity in human neuroblastoma-derived SH-SY5Y cells, which are considered an in-vitro model of PD. Dihydromyricetin (DHM), a flavonoid derived from Asian medicinal plant, has also shown effectiveness against oxidative damage and neuroinflammation, hallmarks of neurodegenerative diseases. Here we show that pretreatment of SH-SY5Y cells with DHM concentration-dependently prevented SALS-induced toxicity and that a combination of DHM and BUT resulted in a synergistic protection. The effects of both DHM and BUT in turn could be completely blocked by flumazenil (FLU), a GABA(A) antagonist acting at benzodiazepine receptor site, and by bicuculline (BIC), a GABA(A) antagonist acting at orthosteric site. Beta-hydroxybutyrate (BHB), a free fatty acid 3 (FA3) receptor antagonist, also fully blocked the protective effect of DHM. BHB was shown previously to only partially block the protective effect of BUT. Thus, there are some overlaps and some distinct differences in protective mechanisms of DHM and BUT against SALS-induced toxicity. It is suggested that a combination of DHM and BUT may have therapeutic potential in PD. However, further in-vivo verifications are necessary.
Salsolinol Induces Parkinson's Disease Through Activating NLRP3-Dependent Pyroptosis and the Neuroprotective Effect of Acteoside.[Pubmed:36454451]
Neurotox Res. 2022 Dec;40(6):1948-1962.
Endogenous neurotoxin 1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroiso-quinoline (Salsolinol, SAL) is a dopamine metabolite that is toxic to dopaminergic neurons in vitro and in vivo, and is involved in the pathogenesis of Parkinson's disease (PD). However, the molecular mechanism by which SAL induces neurotoxicity in PD remains challenging for future investigations. This study found that SAL induced neurotoxicity in SH-SY5Y cells and mice. RNA sequencing (RNAseq) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were used to detect differentially expressed genes in SAL-treated SH-SY5Y cells. We found that NLR family pyrin domain-containing 3 (NLRP3)-dependent pyroptosis was enriched by SAL, which was validated by in vitro and in vivo SAL models. Further, NLRP3 inflammasome-related genes (ASC, NLRP3, active caspase 1, IL-1beta, and IL-18) were increased at the mRNA and protein level. Acteoside mitigates SAL-induced neurotoxicity by inhibiting NLRP3 inflammasome-related pyroptosis in in vitro and in vivo PD models. In summary, the present study suggests for the first time that NLRP3-dependent pyroptosis plays a role in the pathogenesis of SAL-induced PD, and acteoside mitigates SAL-induced pyroptosis-dependent neurotoxicity in in vitro and in vivo PD models. The present results demonstrated a new mechanism whereby SAL mediates neurotoxicity by activating NLRP3-dependent pyroptosis, further highlighting SAL-induced pyroptosis-dependent neurotoxicity as a potential therapeutic target in PD.


