AnthroneCAS# 90-44-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 90-44-8 | SDF | Download SDF |
| PubChem ID | 7018.0 | Appearance | Powder |
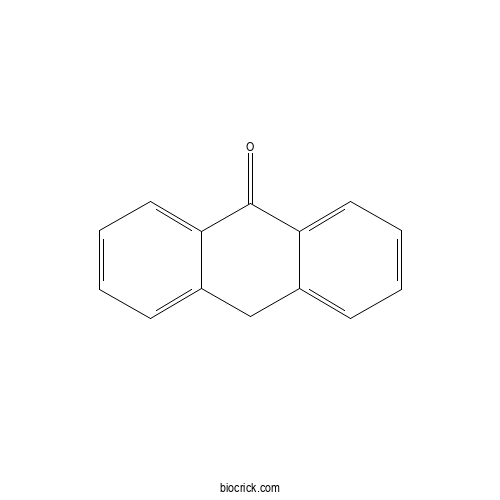
| Formula | C14H10O | M.Wt | 194.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 10H-anthracen-9-one | ||
| SMILES | C1C2=CC=CC=C2C(=O)C3=CC=CC=C31 | ||
| Standard InChIKey | RJGDLRCDCYRQOQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10O/c15-14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-8H,9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Anthrone Dilution Calculator

Anthrone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1485 mL | 25.7427 mL | 51.4854 mL | 102.9707 mL | 128.7134 mL |
| 5 mM | 1.0297 mL | 5.1485 mL | 10.2971 mL | 20.5941 mL | 25.7427 mL |
| 10 mM | 0.5149 mL | 2.5743 mL | 5.1485 mL | 10.2971 mL | 12.8713 mL |
| 50 mM | 0.103 mL | 0.5149 mL | 1.0297 mL | 2.0594 mL | 2.5743 mL |
| 100 mM | 0.0515 mL | 0.2574 mL | 0.5149 mL | 1.0297 mL | 1.2871 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- limocitrin -3-O-rutinoside
Catalog No.:BCX0717
CAS No.:79384-27-3
- Prunetrin
Catalog No.:BCX0716
CAS No.:154-36-9
- Salsolinol
Catalog No.:BCX0715
CAS No.:27740-96-1
- Bufotenidine
Catalog No.:BCX0714
CAS No.:487-91-2
- Epigallocatechin gallate octaacetate
Catalog No.:BCX0713
CAS No.:148707-39-5
- Oleandrin,anhydro-16-deacetyl-
Catalog No.:BCX0712
CAS No.:69549-58-2
- Pinolenic acid
Catalog No.:BCX0711
CAS No.:16833-54-8
- Digitoxigenin
Catalog No.:BCX0710
CAS No.:143-62-4
- Oleandrigenin
Catalog No.:BCX0709
CAS No.:465-15-6
- Oroxylin A-7-glucoside
Catalog No.:BCX0708
CAS No.:36948-77-3
- 5-Hydroxy-2′,3,4′,7-tetramethoxyflavone
Catalog No.:BCX0707
CAS No.:19056-75-8
- Methyl jasmonate
Catalog No.:BCX0706
CAS No.:1211-29-6
- D-Lyxose
Catalog No.:BCX0719
CAS No.:1114-34-7
- Ligupurpuroside D
Catalog No.:BCX0720
CAS No.:1194056-35-3
- 7-Ketodeoxycholic acid
Catalog No.:BCX0721
CAS No.:911-40-0
- Rabdosiin
Catalog No.:BCX0722
CAS No.:119152-54-4
- Melibiose
Catalog No.:BCX0723
CAS No.:585-99-9
- Neoanhydropodophyllol
Catalog No.:BCX0724
CAS No.:62287-47-2
- (+)-Rabdosiin
Catalog No.:BCX0725
CAS No.:263397-69-9
- 6-Methoxy-2-(2-phenylethyl)chromone
Catalog No.:BCX0726
CAS No.:84294-89-3
- 6-Methoxy-2-[2-(3'-methoxyphenyl)ethyl]chromone
Catalog No.:BCX0727
CAS No.:84294-88-2
- Melezitose
Catalog No.:BCX0728
CAS No.:597-12-6
- 3'-Hydroxypterostilbene
Catalog No.:BCX0729
CAS No.:475231-21-1
- 6,7-Dimethoxy-2-[2-(4'-methoxyphenyl)ethyl]chromone
Catalog No.:BCX0730
CAS No.:117596-92-6
Characterizations of biogenic selenium nanoparticles and their anti-biofilm potential against Streptococcus mutans ATCC 25175.[Pubmed:38626650]
J Trace Elem Med Biol. 2024 Apr 7;84:127448.
INTRODUCTION: S. mutans has been identified as the primary pathogenic bacterium in biofilm-mediated dental caries. The biogenic selenium nanoparticles (SeNPs) produced by L. plantarum KNF-5 were used in this study against S. mutans ATCC 25175. OBJECTIVES: The aims of this study were: (1) the biosynthesis of SeNPs by L. plantarum KNF-5, (2) the characterization of SeNPs, (3) the investigation of the inhibitory effect of biogenic SeNPs against S. mutans ATCC 25175, and (4) the determination of the anti-biofilm potential of SeNPS against S. mutans ATCC 25175. METHODOLOGY: 3 mL of the culture was added to 100 mL of MRS medium and incubated. After 4 h, Na(2)SeO(3) solution (concentration 100 mug/mL) was added and incubated at 37 degrees C for 36 h. The color of the culture solution changed from brownish-yellow to reddish, indicating the formation of SeNPs. The characterization of SeNPs was confirmed by UV-Vis spectrophotometry, FTIR, SEM-EDS and a particle size analyzer. The antibacterial activity was determined by the disk diffusion method, the MIC by the micro-double dilution method, and the biofilm inhibitory potential by the crystal violet method and the MTT assay. The effect of SeNPs on S. mutans ATCC 25175 was determined using SEM and CLSM spectrometry techniques. The sulfate-Anthrone method was used to analyze the effect of SeNPs on insoluble extracellular polysaccharides. The expression of genes in S. mutans ATCC 25175 was analyzed by real-time quantitative polymerase chain reaction (RT-qPCR). PREPARATION OF NANOPARTICLES: SeNPs produced by probiotic bacteria are considered a safe method. In this study, L. plantarum KNF-5 (probiotic strain) was used for the production of SeNPs. RESULTS: The biogenic SeNPs were spherical and coated with proteins and polysaccharides and had a diameter of about 270 nm. The MIC of the SeNPs against S. mutans ATCC 25175 was 3.125 mg/mL. Biofilm growth was also significantly suppressed at this concentration. The expression of genes responsible for biofilm formation (GtfB, GtfC, BrpA and GbpB,) was reduced when S. mutans ATCC 25175 was treated with SeNPs. CONCLUSION: It was concluded that the biogenic SeNPs produced by L. plantarum KNF-5 was highly effective to inhibit the growth of S. mutans ATCC 25175. NOVELTY STATEMENT: The application of biogenic SeNPs, a natural anti-biofilm agent against S. mutans ATCC 25175. In the future, this study will provide a new option for the prevention and treatment of dental caries.
Exploring the Constituents and Mechanisms of Polygonum multiflorum Thunb. in Mitigating Ischemic Stroke: A Network Pharmacology and Molecular Docking Study.[Pubmed:38623977]
Comb Chem High Throughput Screen. 2024 Apr 15.
Polygonum multiflorum Thunb. (PMT) has shown promise in exerting cerebrovascular protective effects, and its potential for treating ischemic stroke (IS) has garnered attention. However, the lack of clarity regarding its chemical constituents and mechanisms has significantly hindered its clinical application. In this study, we employed network pharmacology and molecular docking techniques for the first time to elucidate the potential compounds and targets of PMT in treating IS. The databases CTD, DrugBank, DisGeNET, GeneCards, OMIM, TTD, PGKB, NCBI, TCMIP, CNKI, PubMed, ZINC, STITCH, BATMAN, ETCM and Swiss provided information on targets related to IS and components of PMT, along with their associated targets. We constructed "compound-target" and protein-protein interaction (PPI) networks sourced from the STRING database using the Cytoscape software. Gene Ontology (GO) enrichment analysis and KEGG pathway analysis were conducted using the DAVID database. Molecular docking between core targets and active compounds was conducted using Autodock4 software. Experiments were performed in an oxygen-glucose deprivation and reperfusion (OGD/R) model to validate the anti-IS activity of compounds isolated from PMT preliminarily. Network pharmacological analysis revealed 16 core compounds, including resveratrol, polydatin, TSG, omega- hydroxyemodin, emodin Anthrone, tricin, moupinamide, and others, along with 11 high-degree targets, such as PTGS1, PTGS2, ADORA1, ADORA2, CA1, EGFR, ESR1, ESR2, SRC, MMP3 and MMP9. GO and KEGG enrichment analyses revealed the involvement of HIF-1, Akt signaling pathway and energy metabolism-related signaling pathways. Molecular docking results emphasized eight key compounds and confirmed their interactions with corresponding targets. In vitro OGD/R model experiments identified TSG and tricin as the primary active substances within PMT for its anti-stroke activity. This study contributes new insights into the potential development of PMT for stroke prevention and treatment.


