Epigallocatechin gallate octaacetateCAS# 148707-39-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 148707-39-5 | SDF | Download SDF |
| PubChem ID | 11787645.0 | Appearance | Powder |
| Formula | C38H34O19 | M.Wt | 794.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AcEGCG;Peracetylated epigallocatechin-3-gallate;EGCG Octaacetate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
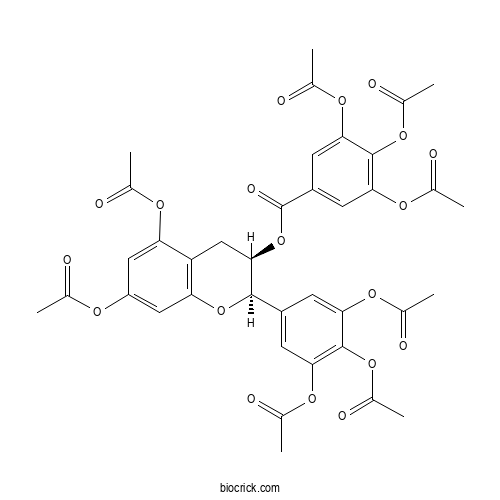
| Chemical Name | [(2R,3R)-5,7-diacetyloxy-2-(3,4,5-triacetyloxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-triacetyloxybenzoate | ||
| SMILES | CC(=O)OC1=CC2=C(CC(C(O2)C3=CC(=C(C(=C3)OC(=O)C)OC(=O)C)OC(=O)C)OC(=O)C4=CC(=C(C(=C4)OC(=O)C)OC(=O)C)OC(=O)C)C(=C1)OC(=O)C | ||
| Standard InChIKey | SVHJCTSSYQPWEV-VSJLXWSYSA-N | ||
| Standard InChI | InChI=1S/C38H34O19/c1-16(39)48-26-13-28(49-17(2)40)27-15-34(57-38(47)25-11-32(52-20(5)43)37(55-23(8)46)33(12-25)53-21(6)44)35(56-29(27)14-26)24-9-30(50-18(3)41)36(54-22(7)45)31(10-24)51-19(4)42/h9-14,34-35H,15H2,1-8H3/t34-,35-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Epigallocatechin gallate octaacetate Dilution Calculator

Epigallocatechin gallate octaacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2584 mL | 6.2919 mL | 12.5838 mL | 25.1677 mL | 31.4596 mL |
| 5 mM | 0.2517 mL | 1.2584 mL | 2.5168 mL | 5.0335 mL | 6.2919 mL |
| 10 mM | 0.1258 mL | 0.6292 mL | 1.2584 mL | 2.5168 mL | 3.146 mL |
| 50 mM | 0.0252 mL | 0.1258 mL | 0.2517 mL | 0.5034 mL | 0.6292 mL |
| 100 mM | 0.0126 mL | 0.0629 mL | 0.1258 mL | 0.2517 mL | 0.3146 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oleandrin,anhydro-16-deacetyl-
Catalog No.:BCX0712
CAS No.:69549-58-2
- Pinolenic acid
Catalog No.:BCX0711
CAS No.:16833-54-8
- Digitoxigenin
Catalog No.:BCX0710
CAS No.:143-62-4
- Oleandrigenin
Catalog No.:BCX0709
CAS No.:465-15-6
- Oroxylin A-7-glucoside
Catalog No.:BCX0708
CAS No.:36948-77-3
- 5-Hydroxy-2′,3,4′,7-tetramethoxyflavone
Catalog No.:BCX0707
CAS No.:19056-75-8
- Methyl jasmonate
Catalog No.:BCX0706
CAS No.:1211-29-6
- Ginsenoside Ra6
Catalog No.:BCX0705
CAS No.:1346522-89-1
- Endothalic acid
Catalog No.:BCX0704
CAS No.:145-73-3
- Epirosmanol
Catalog No.:BCX0703
CAS No.:93380-12-2
- Mussaenosidic acid
Catalog No.:BCX0702
CAS No.:82451-22-7
- Methylcantharidinimide
Catalog No.:BCX0701
CAS No.:76970-78-0
- Bufotenidine
Catalog No.:BCX0714
CAS No.:487-91-2
- Salsolinol
Catalog No.:BCX0715
CAS No.:27740-96-1
- Prunetrin
Catalog No.:BCX0716
CAS No.:154-36-9
- limocitrin -3-O-rutinoside
Catalog No.:BCX0717
CAS No.:79384-27-3
- Anthrone
Catalog No.:BCX0718
CAS No.:90-44-8
- D-Lyxose
Catalog No.:BCX0719
CAS No.:1114-34-7
- Ligupurpuroside D
Catalog No.:BCX0720
CAS No.:1194056-35-3
- 7-Ketodeoxycholic acid
Catalog No.:BCX0721
CAS No.:911-40-0
- Rabdosiin
Catalog No.:BCX0722
CAS No.:119152-54-4
- Melibiose
Catalog No.:BCX0723
CAS No.:585-99-9
- Neoanhydropodophyllol
Catalog No.:BCX0724
CAS No.:62287-47-2
- (+)-Rabdosiin
Catalog No.:BCX0725
CAS No.:263397-69-9
In vitro cytotoxicity of (-)-EGCG octaacetate on MDAMB-231 and SKHep-1 human carcinoma cells: a pharmacological consideration on prodrug design.[Pubmed:19020784]
Int J Mol Med. 2008 Dec;22(6):841-5.
Esterification of acetate with generic pharmaceutical compound has been commonly employed to produce ester prodrug for improving its potency when compared with the mother compound. Acetate, on the other hand, has been recognized to have inhibitory effect on the respiratory biochemistry. Here we demonstrate that acetate at a concentration of 400 microM exhibited significant growth inhibitory activity on two human cancer cell lines, the MDAMB-231 breast cancer and the SKHep-1 hepatoma cell lines. To establish the ester prodrug with multi-acetate ester conjugates as our experimental model, one molecule of (-)-epigallocatechin gallate was required to conjugate with eight molecules of acetate forming the corresponding (-)-Epigallocatechin gallate octaacetate prodrug. Chemical structure of this Epigallocatechin gallate octaacetate ester prodrug was confirmed by both 13C and 1H nuclear magnetic resonance spectra and mass spectrometry. Further cytotoxic assay using both MDAMB-231 and SKHep-1 human carcinoma cell lines showed that acetate at a concentration of 400 microM exhibits an additional cytotoxic effect with (-)-epigallocatechin gallate at a concentration of 50 microM, although the additional effect was not as high as (-)-Epigallocatechin gallate octaacetate ester prodrug alone at a concentration of 50 microM. Our results thus raise a pharmacological consideration of using multi-acetate conjugate as the ester prodrug where the release of free acetate by esterase could be part of the explanation for the improved in vitro cytotoxicity.


