Mussaenosidic acidCAS# 82451-22-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82451-22-7 | SDF | Download SDF |
| PubChem ID | 133556235.0 | Appearance | Powder |
| Formula | C16H24O10 | M.Wt | 376.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
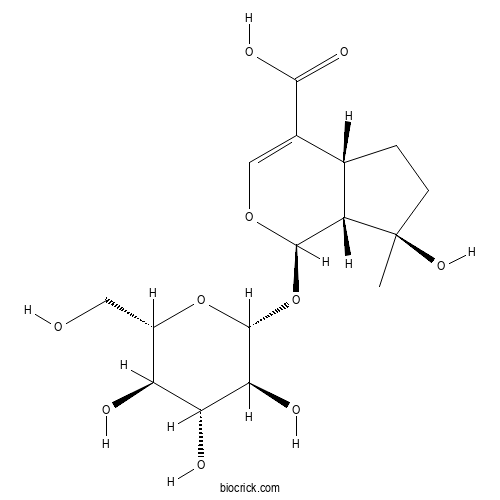
| Chemical Name | (1R,4aR,7R,7aR)-7-hydroxy-7-methyl-1-[(2R,3S,4R,5R,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4a,5,6,7a-tetrahydro-1H-cyclopenta[c]pyran-4-carboxylic acid | ||
| SMILES | CC1(CCC2C1C(OC=C2C(=O)O)OC3C(C(C(C(O3)CO)O)O)O)O | ||
| Standard InChIKey | VLCHQFXSBHIBRV-KZFODJCISA-N | ||
| Standard InChI | InChI=1S/C16H24O10/c1-16(23)3-2-6-7(13(21)22)5-24-14(9(6)16)26-15-12(20)11(19)10(18)8(4-17)25-15/h5-6,8-12,14-15,17-20,23H,2-4H2,1H3,(H,21,22)/t6-,8-,9-,10-,11+,12-,14+,15+,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Mussaenosidic acid Dilution Calculator

Mussaenosidic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.657 mL | 13.2852 mL | 26.5703 mL | 53.1406 mL | 66.4258 mL |
| 5 mM | 0.5314 mL | 2.657 mL | 5.3141 mL | 10.6281 mL | 13.2852 mL |
| 10 mM | 0.2657 mL | 1.3285 mL | 2.657 mL | 5.3141 mL | 6.6426 mL |
| 50 mM | 0.0531 mL | 0.2657 mL | 0.5314 mL | 1.0628 mL | 1.3285 mL |
| 100 mM | 0.0266 mL | 0.1329 mL | 0.2657 mL | 0.5314 mL | 0.6643 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methylcantharidinimide
Catalog No.:BCX0701
CAS No.:76970-78-0
- Cauloside D
Catalog No.:BCX0700
CAS No.:12672-45-6
- Cyclanoline
Catalog No.:BCX0699
CAS No.:18556-27-9
- Myricetin 3-O-rutinoside
Catalog No.:BCX0698
CAS No.:41093-68-9
- Desacylsenegasaponin B
Catalog No.:BCX0697
CAS No.:163589-51-3
- Quercetin 3-O-[beta-D-xylosyl-(1->2)-beta-D-glucoside]
Catalog No.:BCX0696
CAS No.:83048-35-5
- Cavidine
Catalog No.:BCX0695
CAS No.:32728-75-9
- Polygalasaponin XXVIII
Catalog No.:BCX0694
CAS No.:176182-01-7
- N-benzyl-heptadecanamide
Catalog No.:BCX0693
CAS No.:883715-19-3
- N-(3-methoxybenzyl)-octadecanamide
Catalog No.:BCX0692
CAS No.:1429659-99-3
- 8,9-epoxy-3,10-diisobutyryloxythymol
Catalog No.:BCX0691
CAS No.:22518-06-5
- Farnesene
Catalog No.:BCX0690
CAS No.:502-61-4
- Epirosmanol
Catalog No.:BCX0703
CAS No.:93380-12-2
- Endothalic acid
Catalog No.:BCX0704
CAS No.:145-73-3
- Ginsenoside Ra6
Catalog No.:BCX0705
CAS No.:1346522-89-1
- Methyl jasmonate
Catalog No.:BCX0706
CAS No.:1211-29-6
- 5-Hydroxy-2′,3,4′,7-tetramethoxyflavone
Catalog No.:BCX0707
CAS No.:19056-75-8
- Oroxylin A-7-glucoside
Catalog No.:BCX0708
CAS No.:36948-77-3
- Oleandrigenin
Catalog No.:BCX0709
CAS No.:465-15-6
- Digitoxigenin
Catalog No.:BCX0710
CAS No.:143-62-4
- Pinolenic acid
Catalog No.:BCX0711
CAS No.:16833-54-8
- Oleandrin,anhydro-16-deacetyl-
Catalog No.:BCX0712
CAS No.:69549-58-2
- Epigallocatechin gallate octaacetate
Catalog No.:BCX0713
CAS No.:148707-39-5
- Bufotenidine
Catalog No.:BCX0714
CAS No.:487-91-2
Iridoid glucosides in the genus Veronica (Plantaginaceae) from New Zealand.[Pubmed:28550715]
Phytochemistry. 2017 Aug;140:174-180.
Four simple iridoid glucosides, three known esters of catalpol, seven esters of aucubin, and two phenylethanoids were isolated from Veronica hookeri (syn. Hebe ciliolata; Plantaginaceae). Of these, none of four aromatic (p-methoxybenzoyl, isovanilloyl, veratroyl, caffeoyl) 6-O-esters of aucubin and 6''-O-benzoyl Mussaenosidic acid, had been reported from nature before. Similarly, three simple iridoid glucosides, two esters of 6-O-rhamnopyranosylcatapol, and two phenylethanoid glucosides, as well as 1-O-benzoyl-3-alpha-glucuronosylglycerol, and 1-O-beta-benzoyl rutinoside were isolated from Veronica pinguifolia (syn. Hebe pinguifolia). The compound 3''-O-benzoyl-2''-O-caffeoyl 6-O-rhamnopyranosylcatalpol had not been reported previously. The pattern of the structural features of the iridoid glucosides is overlaid onto the latest molecular phylogenetic framework of Veronica sects. Hebe and Labiatoides, and discussed in the context of evolutionary trends.
In Vitro and In Silico Antidiabetic and Antimicrobial Evaluation of Constituents from Kickxia ramosissima (Nanorrhinum ramosissimum).[Pubmed:28507520]
Front Pharmacol. 2017 May 1;8:232.
Background and Aims:Kickxia ramosissima (Wall.) Janch (or Nanorrhinum ramosissimum (Wall.) Betsche is a well-known medicinal plant in Pakistan that is traditionally used in diabetic and inflammatory conditions. Because little information is available on its phytochemical composition, a range of constituents were isolated and evaluated in vitro in assays related to the traditional use. Methods: Dried whole plant material was extracted and chromatographically fractionated. Isolated constituents were evaluated in silico and in vitro in assays related to the traditional use against diabetes (inhibition of alpha-glucosidase activity; inhibition of advanced glycation endproducts) and in inflammatory conditions (inhibition of AAPH induced linoleic acid peroxidation, inhibition of 15-LOX, antimicrobial activity). Results: Phytochemical analysis of the extracts and fractions led to isolation of 7 compounds, including the iridoids kickxiasine (being a new compound), Mussaenosidic acid, mussaenoside and linarioside; the flavonoids pectolinarigenin and pectolinarin; and 4-hydroxy-benzoic acid methyl ester. The iridoids showed weak antiglycation activity. The flavonoids, however, showed interesting results as pectolinarigenin was highly active compared to pectolinarin. In the alpha-glucosidase inhibition assay, only weak activity was observed for the iridoids. However, the flavonoid pectolinarigenin showed good activity, followed by pectolinarin. In the 15-LOX experiment, moderate inhibition was recorded for most compounds, the iridoids Mussaenosidic acid and mussaenoside being the most active. In the AAPH assay, weak or no inhibition was recorded for all compounds. The in silico assays for the alpha-glucosidase and 15-LOX assays confirmed the results of respective in vitro assays. Pectolinarigenin showed moderate antimicrobial activity against Staphylococcus aureus, Plasmodium falciparum K1, and Trypanosoma cruzi, but it was not cytotoxic on a human MRC-5 cell line. Conclusion: Our findings may in part contribute to explain the traditional use of K. ramosissima.
Secoiridoids and other chemotaxonomically relevant compounds in Pedicularis: phytochemical analysis and comparison of Pedicularis rostratocapitata Crantz and Pedicularis verticillata L. from Dolomites.[Pubmed:26828611]
Nat Prod Res. 2016 Aug;30(15):1698-705.
We compared the respective metabolite patterns of two Pedicularis species from Dolomites. Seven phenylethanoid glycosides, i.e., verbascoside (1), echinacoside (2), angoroside A (3), cistantubuloside B1 (4), wiedemannioside C (5), campneoside II (11) and cistantubuloside C1 (12), together with several iridoid glucosides as aucubin (6), euphroside (7), monomelittoside (8), Mussaenosidic acid (9) and 8-epiloganic acid (13) were identified. Pedicularis verticillata showed also the presence of greatly unexpected secoiridoids, ligustroside (14) and excelside B (15), very rare compounds in Lamiales. Both PhGs and iridoids are considered of taxonomical relevance in the Asteridae and their occurrence in Pedicularis was discussed. In particular, the exclusive presence of several compounds such as 8-epiloganic acid (13), campneoside II (11), cistantubuloside C1 (12), ligustroside (14) and excelside B (15) in Pedicularis rostratocapitata, and angoroside A (3), cistantubuloside B1 (4) and wiedemannioside C (5) in P. verticillata could be considered specific markers for the two botanical entities.
Iridoids and phenylethanoid from Pedicularis kerneri Dalla Torre growing in Dolomites, Italy.[Pubmed:26207992]
Nat Prod Res. 2016;30(3):327-31.
In this study, we report the first phytochemical analysis of polar fraction of Pedicularis kerneri Dalla Torre growing in Dolomites, Italy. Several iridoid glucosides were isolated, namely aucubin (1), monomelittoside (2), plantarenaloside (3), euphroside (4), Mussaenosidic acid (5) and 8-epiloganic acid (6), showing a composition in accordance with previous study on this genus. The studied samples, collected from Dolomites, presented a chemotype already recognised in species from North America, characterised by euphroside (4) and aucubin (1) as main components, but the main character was the presence of monomelittoside (2) never reported in this genus. The identification of verbascoside (7), leucosceptoside A (9) and echinacoside (10) complete the systematic framing of this species since is ascertained the co-occurrence of phenylethanoid glycosides with iridoids in Lamiales species.
Evaluation of anti-HIV-1 activity of a new iridoid glycoside isolated from Avicenna marina, in vitro.[Pubmed:25239814]
Int Immunopharmacol. 2014 Nov;23(1):262-6.
This study was carried out to check the efficacy of methanol seed extract of Avicenna marina and its column chromatographic fractions on Peripheral Blood Mono nuclear Cells (PBMCs) toxicity and HIV-1 replication. The anti-HIV-1 activities of crude methanol extract and its fractions were performed by use of real-time polymerase chain reaction (PCR) assay and HIV-1 p24 antigen kit. A time of drug addiction approach was also done to identify target of anti-HIV compound. The activity of the extracts on CD4, CD3, CD19 and CD45 expression in lymphocytes population was performed by use of flow cytometry. The most active anti-HIV agent was detected by spectroscopic analysis as 2'-O-(4-methoxycinnamoyl) Mussaenosidic acid. The apparent effective concentrations for 50% virus replication (EC50) of methanol extract and iridoid glycoside were 45 and 0.1 mug/ml respectively. The iridoid glycoside also did not have any observable effect on the proportion of CD4, CD3, CD19 and CD45 cells or on the intensity of their expressions on PBMCs. In addition, the expression level of C-C chemokine receptor type 5 (CCR5) and chemokine receptor type 4 (CXCR4) on CD4(+) T cells were decreased in cells treated with this iridoid glycoside. The reduction of these two HIV coreceptors and the result of time of addition study demonstrated that this iridoid glycoside restricts HIV-1 replication on the early stage of HIV infection.
A proposed biosynthetic pathway of picrosides linked through the detection of biochemical intermediates in the endangered medicinal herb Picrorhiza kurroa.[Pubmed:23696248]
Phytochem Anal. 2013 Nov-Dec;24(6):598-602.
INTRODUCTION: Picrorhiza kurroa Royle ex Benth is an important medicinal herb used in the preparation of several herbal drug formulations due to the presence of picroside-I (P-I) and picroside-II (P-II) along with other iridoid-glucosides derivatives. OBJECTIVE: The endangered status of P. kurroa coupled with lack of information on biosynthesis of P-I and P-II necessitate deciphering the biosynthetic pathway for picrosides. METHODS: LC with electrospray ionisation (ESI) and quadrupole time of flight combined with MS/MS was used to detect intermediates and assemble the picrosides biosynthetic pathway in P. kurroa. RESULTS: The presence of catalpol and aucubin, the major backbone structures of picrosides, along with intermediate metabolites boschnaloside, bartsioside and Mussaenosidic acid, was confirmed in ESI negative mode with pseudomolecular ion peaks, that is, m/z 361, m/z 343, m/z 345, m/z 329 and m/z 375 ions and their fragmentation patterns. CONCLUSION: The picrosides biosynthetic pathway is expected to provide a reliable platform towards understanding the molecular components (genes/enzymes) of P-I and P-II biosynthesis in P. kurroa for their eventual utilisation in various applications.
Iridoids from Bellardia trixago (L.) All.[Pubmed:23298403]
Nat Prod Res. 2013 Aug;27(15):1413-6.
The phytochemical study of the polar fraction of Bellardia trixago (L.) All. led to the isolation of eight iridoid glucosides. Five of these glucosides (aucubin (1), bartsioside (2), melampyroside (3), mussaenoside (4) and gardoside methyl ester (5)) were confirmed as they were previously isolated from this plant, and the remaining three known compounds (Mussaenosidic acid (6), geniposidic acid (7) and 8-epiloganin (8)) were isolated here for the first time. Of particular interest were the presence of 7 and 8 due to two reasons: the first one because it is not accompanied with geniposide, the corresponding methyl ester, as in the case of 4 and 6, and the second one because it is the parent compound of iridoids characteristic of Orobanchaceae family. Also an alditol, D-mannitol (9), was recognised for the first time from this species.
Minor iridoids from Scutellaria albida ssp. albida. Inhibitory potencies on lipoxygenase, linoleic acid lipid peroxidation and antioxidant activity of iridoids from Scutellaria sp.[Pubmed:22630074]
J Enzyme Inhib Med Chem. 2013 Aug;28(4):704-10.
A new iridoid glycoside, 6'-O-E-caffeoyl-Mussaenosidic acid , in addition to one known aglycon, four known triterpenes and one known flavonoid, were isolated from the aerial parts of Scutellaria albida subsp. albida. Furthermore, 12 iridoids with similar structures isolated from Scutellaria sp., were examined for their inhibitory potency on lipoxygenase and lipid peroxidation, as well as their antioxidant activity, in comparison to known antioxidants e.g. caffeic acid, nordihydroguaretic acid (NDGA) and trolox. AAPH, DPPH and soybean lipoxygenase (LOX) assays were used for the tests. This investigation led to interesting observations considering the Structure-Activity Relationship. According to our results, the presence of a p-coumaroyl group optimized and even dramatically changed the biological responses of the investigated iridoids.
Validated high performance thin layer chromatographic method for simultaneous quantification of major iridoids in Vitex trifolia and their antioxidant studies.[Pubmed:22226914]
J Pharm Biomed Anal. 2012 Mar 5;61:207-14.
Negundoside (1), agnuside (2) and 6'-p-hydroxy benzoyl Mussaenosidic acid (3) are known bioactive metabolites in Vitex trifolia. In the present study a simple precise and reproducible method was developed for simultaneous quantitation of NS (1), AS (2) and HMA (3) and the antioxidant capacity of above markers has also been determined. Marker compounds have been resolved using silica gel 60 F(254) plates, petroleum ether (60-80)/toluene/acetone/water (10:10:80:2 v/v/v/v) as the mobile phases. The method does not employ any derivatisation procedure and can be used as a quality control tool for routine analysis of drugs V. trifolia and V. negundo together with their commercial extracts. NS (1), AS (2) and HMA (3) showed significant activity in DPPH and NO radical scavenging assays.
HCV-NS3/4A protease inhibitory iridoid glucosides and dimeric foliamenthoic acid derivatives from Anarrhinum orientale.[Pubmed:21506603]
J Nat Prod. 2011 May 27;74(5):943-8.
Four new compounds were isolated from the methanol extract of the aerial parts of Anarrhinum orientale: 6'-O-cinnamoylMussaenosidic acid (1), 6'-O-cinnamoyl-8-O-(6'''-O-cinnamoylglucopyranosyl)Mussaenosidic acid (2), (2E,6E)-8-[(2E,6E)-8-hydroxy-2,6-dimethylocta-2,6-dienoyl]oxy-2,6-dimethylocta-2,6-dienoic acid (3), and (2E,6E)-8-[(2E,6E)-8-acetoxy-2,6-dimethylocta-2,6-dienoyl]oxy-2,6-dimethylocta-2,6-dienoic acid (4). The known 8-O-cinnamoylMussaenosidic acid (5) was also identified. All five compounds were tested for inhibition of the hepatitis C virus (HCV) protease. Compounds 1 and 5 exhibited moderate activity, while 2 and 3 showed weak effects. No inhibitory activity on the human serine protease was observed for any of these compounds, which may infer the selectivity toward the viral protease. A computational docking study of the isolated compounds against HCV protease was used to formulate a hypothetical mechanism for the inhibitory activity of the active compounds on the enzymes tested.


