L-5-HydroxytryptophanCAS# 4350-09-8 |
- DL-5-Hydroxytryptophan
Catalog No.:BCN1232
CAS No.:56-69-9
- 5-Hydroxytryptophan
Catalog No.:BCX0905
CAS No.:114-03-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4350-09-8 | SDF | Download SDF |
| PubChem ID | 439280 | Appearance | White-grey powder |
| Formula | C11H12N2O3 | M.Wt | 220 |
| Type of Compound | Nitrogen-containing Compounds | Storage | Desiccate at -20°C |
| Synonyms | L-5-HTP | ||
| Solubility | DMSO : 100 mg/mL (454.09 mM; Need ultrasonic) | ||
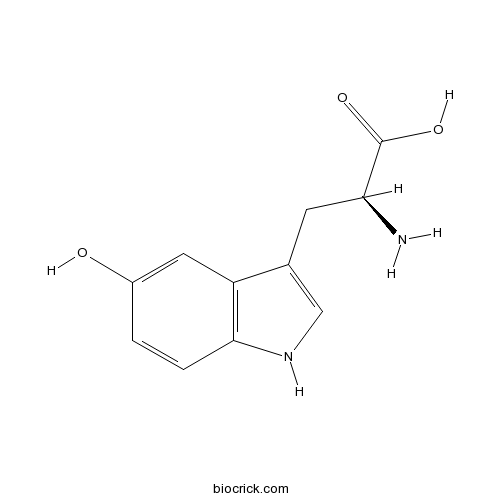
| Chemical Name | (2S)-2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid | ||
| SMILES | C1=CC2=C(C=C1O)C(=CN2)CC(C(=O)O)N | ||
| Standard InChIKey | LDCYZAJDBXYCGN-VIFPVBQESA-N | ||
| Standard InChI | InChI=1S/C11H12N2O3/c12-9(11(15)16)3-6-5-13-10-2-1-7(14)4-8(6)10/h1-2,4-5,9,13-14H,3,12H2,(H,15,16)/t9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

L-5-Hydroxytryptophan Dilution Calculator

L-5-Hydroxytryptophan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5455 mL | 22.7273 mL | 45.4545 mL | 90.9091 mL | 113.6364 mL |
| 5 mM | 0.9091 mL | 4.5455 mL | 9.0909 mL | 18.1818 mL | 22.7273 mL |
| 10 mM | 0.4545 mL | 2.2727 mL | 4.5455 mL | 9.0909 mL | 11.3636 mL |
| 50 mM | 0.0909 mL | 0.4545 mL | 0.9091 mL | 1.8182 mL | 2.2727 mL |
| 100 mM | 0.0455 mL | 0.2273 mL | 0.4545 mL | 0.9091 mL | 1.1364 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- K 41498
Catalog No.:BCC5867
CAS No.:434938-41-7
- Dacarbazine
Catalog No.:BCC1174
CAS No.:4342-03-4
- Nandrolone
Catalog No.:BCC9086
CAS No.:434-22-0
- Lithocholic Acid
Catalog No.:BCC3805
CAS No.:434-13-9
- Oxymetholone
Catalog No.:BCC4692
CAS No.:434-07-1
- Methenolone acetate
Catalog No.:BCC9028
CAS No.:434-05-9
- Ethisterone
Catalog No.:BCC4478
CAS No.:434-03-7
- VU 0357121
Catalog No.:BCC4595
CAS No.:433967-28-3
- 3-O-Acetyloleanolic acid
Catalog No.:BCN5486
CAS No.:4339-72-4
- Toddaculine
Catalog No.:BCN3639
CAS No.:4335-12-0
- Boc-Tyr-OMe
Catalog No.:BCC3459
CAS No.:4326-36-7
- 6-Hydroxykaempferol
Catalog No.:BCN3334
CAS No.:4324-55-4
- H-Arg(Tos)-OH
Catalog No.:BCC2867
CAS No.:4353-32-6
- 5-Hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one
Catalog No.:BCC8350
CAS No.:4354-76-1
- (-)-Curine
Catalog No.:BCN2673
CAS No.:436-05-5
- Diffractic Acid
Catalog No.:BCN8506
CAS No.:436-32-8
- Fangchinoline
Catalog No.:BCN5956
CAS No.:436-77-1
- Ajmaline
Catalog No.:BCN3867
CAS No.:4360-12-7
- JKC 363
Catalog No.:BCC6022
CAS No.:436083-30-6
- Kobe0065
Catalog No.:BCC5290
CAS No.:436133-68-5
- Tetrodotoxin
Catalog No.:BCN1035
CAS No.:4368-28-9
- MRS 2365
Catalog No.:BCC5879
CAS No.:436847-09-5
- Gentisin
Catalog No.:BCN7518
CAS No.:437-50-3
- Genkwanin
Catalog No.:BCN5488
CAS No.:437-64-9
Exposure to Acute and Chronic Fluoxetine has Differential Effects on Sociability and Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus of Juvenile Male BALB/c Mice.[Pubmed:29940216]
Neuroscience. 2018 Aug 21;386:1-15.
Although the neurobiological mechanisms underlying autism spectrum disorder (ASD) are still unknown, dysregulation of serotonergic systems has been implicated in the etiology of ASD, and serotonergic antidepressant drugs are often prescribed to treat some symptoms of ASD. The BALB/c strain of mice express a dysregulated serotonergic system and a phenotype that is relevant to ASD. In this study, juvenile male BALB/c mice were exposed to the selective serotonin reuptake inhibitor fluoxetine either chronically (18mg/kg/day in drinking water, post-natal day (PND) 28-39) or acutely (18mg/kg, i.p.; PND40), or to vehicle control conditions (0.9% sterile saline, i.p.; PND40), prior to being exposed to the three-chambered sociability test (SAT; PND40). One cohort of mice then received an injection of the aromatic amino acid decarboxylase inhibitor, NSD-1015, and one hour later brain tissue was collected for quantification of 5-hydroxytryptophan accumulation in the dorsal raphe nucleus (DR) as a measure of TPH2 activity. For the second cohort, brain tissue was collected ninety minutes after the onset of the social phase of the SAT and prepared for immunohistochemical staining for c-Fos and TPH2 to measure the activation of serotonergic neurons within subregions of the DR. Acute fluoxetine decreased social behavior, while chronic fluoxetine increased social behavior compared with vehicle-treated controls. Furthermore, acute and chronic fluoxetine treatments were without effect on TPH2 activity but differentially affected populations of serotonergic neurons in the DR. These data are consistent with the hypothesis that serotonergic systems are implicated in social behavior that is relevant for ASD.
Neuronal Development in the Larvae of the Invasive Biofouler Dreissena polymorpha (Mollusca: Bivalvia), with Special Attention to Sensory Elements and Swimming Behavior.[Pubmed:29949436]
Biol Bull. 2018 Jun;234(3):192-206.
Although understanding of the neuronal development of Trochozoa has progressed recently, little attention has been paid to freshwater bivalves, including species with a strong ecological impact, such as the zebra mussel (Dreissena polymorpha). Therefore, an important question might concern how the developing nervous system is involved in the formation of the rapid and successful invasive behavior of this species. Our aim was to reveal the neuronal development of trochophore and veliger larvae of Dreissena, with special attention to the organization of sensory structures and their possible involvement in detecting environmental cues. After applying serotonin and FMRFamide immunocytochemistry, the first serotonin immunoreactive sensory elements appeared 16-18 hours after fertilization, whereas the first FMRFamide immunoreactive sensory cell was seen only at 32 hours of development (trochophore stage). Later, sensory elements were found in three parts of the larval body, including the apical organ, the posterior region, and the stomach. Although differences in the timing of appearance and the morphology of cells were observed, the two signaling systems showed basic similarity in their organization pattern until the end of the veliger stage. Pharmacological, physiological, and quantitative immunocytochemical investigations were also performed, suggesting the involvement of both the serotoninergic system and the FMRFamidergic system in sensomotor processes. Manipulation of the serotonin synthesis by para-chloroplenylalanine and 5-hydroxytryptophane, as well as application of increased salinity, influenced larval swimming activity, both accompanied by changes in immunofluorescence intensity. We concluded that these two early sensory systems may play an important role in the development of settlement competency of this biofouling invasive bivalve, Dreissena.
Aptamer Recognition of Multiplexed Small-Molecule-Functionalized Substrates.[Pubmed:29851335]
ACS Appl Mater Interfaces. 2018 Jul 18;10(28):23490-23500.
Aptamers are chemically synthesized oligonucleotides or peptides with molecular recognition capabilities. We investigated recognition of substrate-tethered small-molecule targets, using neurotransmitters as examples, and fluorescently labeled DNA aptamers. Substrate regions patterned via microfluidic channels with dopamine or l-tryptophan were selectively recognized by previously identified dopamine or l-tryptophan aptamers, respectively. The on-substrate dissociation constant determined for the dopamine aptamer was comparable to, though, slightly greater than the previously determined solution dissociation constant. Using prefunctionalized neurotransmitter-conjugated oligo(ethylene glycol) alkanethiols and microfluidics patterning, we produced multiplexed substrates to capture and to sort aptamers. Substrates patterned with l-3,4-dihydroxyphenylalanine, l- threo-dihydroxyphenylserine, and L-5-Hydroxytryptophan enabled comparison of the selectivity of the dopamine aptamer for different targets via simultaneous determination of in situ binding constants. Thus, beyond our previous demonstrations of recognition by protein binding partners (i.e., antibodies and G-protein-coupled receptors), strategically optimized small-molecule-functionalized substrates show selective recognition of nucleic acid binding partners. These substrates are useful for side-by-side target comparisons and future identification and characterization of novel aptamers targeting neurotransmitters or other important small molecules.
Two combined amino acids promote sleep activity in caffeine-induced sleepless model systems.[Pubmed:29854326]
Nutr Res Pract. 2018 Jun;12(3):208-214.
BACKGROUND/OBJECTIVES: The aim of this study was to evaluate the biological and sleep-promoting effects of combined gamma-aminobutyric acid (GABA) and 5-hydroxytryptophan (5-HTP) using caffeine-induced sleepless fruit flies, ICR mice, and Sprague-Dawley rats. MATERIALS/METHODS: Video-tracking analysis was applied to investigate behavioral changes of Drosophila melanogaster. Pentobarbital-induced sleep test and electroencephalogram (EEG) patterns were used for analysis of sleep latency, duration, and quantity and quality of sleep in vertebrate models. RESULTS: Administration of combined GABA/5-HTP could significantly reverse the caffeine induced total distance of flies (P < 0.001). Also, individually administered and combined GABA/5-HTP significantly increased the total sleeping time in the caffeine-induced sleepless ICR mice (P < 0.001). In the caffeine-induced sleepless SD-rats, combined GABA/5-HTP showed significant differences in sleep quality between individual amino acid administrations (P < 0.05). CONCLUSIONS: Taken together, we identified inhibitory effects of combined GABA/5-HTP in locomotor activity, sleep quantity and quality in caffeine-induced sleepless models, indicating that combined GABA/5-HTP may be effective in patients with insomnia by providing sufficient sleep.
Treatment-emergent hypomania possibly associated with over-the-counter supplements.[Pubmed:29955516]
Ment Health Clin. 2018 Mar 26;7(4):160-163.
The use of complementary and alternative medicine (CAM) is gaining popularity in the Western world. Among the general public, CAM is often perceived to be associated with less stigma, fewer adverse effects, and may be more affordable. A number of patients utilize CAM for the treatment of depression; however, as there is limited scientific evidence, the safety profile of these supplements are largely unknown. In this case, a 42-year-old man developed hypomania approximately 1 week after S-adenosylmethionine (SAMe) and 5-hydroxytryptophan (5-HTP) therapy was initiated for depression. The combination of SAMe and 5-HTP can potentially induce hypomanic episodes.
Social approach, anxiety, and altered tryptophan hydroxylase 2 activity in juvenile BALB/c and C57BL/6J mice.[Pubmed:29935278]
Behav Brain Res. 2019 Feb 1;359:918-926.
Autism spectrum disorder (ASD) is a heterogeneous and highly heritable condition with multiple aetiologies. Although the biological mechanisms underlying ASD are not fully understood, evidence suggests that dysregulation of serotonergic systems play an important role in ASD psychopathology. Preclinical models using mice with altered serotonergic neurotransmission may provide insight into the role of serotonin in behaviours relevant to clinical features of ASD. For example, BALB/c mice carry a loss-of-function single nucleotide polymorphism (SNP; C1473G) in tryptophan hydroxylase 2 (Tph2), which encodes the brain-specific isoform of the rate-limiting enzyme for serotonin synthesis, and these mice frequently have been used to model symptoms of ASD. In this study, juvenile male BALB/c (G/G; loss-of-function variant) and C57BL/6J (C/C; wild type variant) mice, were exposed to the three-chamber sociability test, and one week later to the elevated plus-maze (EPM). Tryptophan hydroxylase 2 (TPH2) activity was measured following injection of the aromatic amino acid decarboxylase (AADC)-inhibitor, NSD-1015, and subsequent HPLC detection of 5-hydroxytryptophan (5-HTP) within subregions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). The BALB/c mice showed reduced social behaviour and increased anxious behaviour, as well as decreased 5-HTP accumulation in the rostral and mid-rostrocaudal DR. In the full cohort of mice, TPH2 activity in the mid-rostrocaudal DR was correlated with anxious behaviour in the EPM, however these correlations were not statistically significant within each strain, suggesting that TPH2 activity was not directly associated with either anxiety or sociability. Further research is therefore required to more fully understand how serotonergic systems are involved in mouse behaviours that resemble some of the clinical features of ASD.
[Metabolic analysis of serotonin system in serum and gastric tissues of ovalbumin-induced allergic mice].[Pubmed:29973322]
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018 Apr;34(4):320-326.
Objective To investigate the changes of 5-HT (serotonin) signaling system in allergic diarrhea mice sensitized with ovalbumin (OVA). Methods The seven-to-eight-week-old BALB/c female mice were randomly divided into model group, sodium chromate group and negative control group. The model group and sodium chromate group were intraperitoneally injected with OVAI (50 mug per mouse) at day 0 and day 14 respectively. And starting from the 28th day, OVAII was orally administered (50 mg per mousee) every other day (8 times in total), and the sodium chromate group was given the sodium chromate (78.0 mg/kg) before the oral administration of OVA every other day (8 times in total). The allergic symptoms, including the systemic score, faeces score and body temperature were recorded following the OVA administration for sensitization. The mice were executed 43 days later. Eyeball blood sample was collected, and then serum was seperated by centrifugation, the gastric tissues was taken out. The serum OVA-specific IgE (OVA-SIgE) was detected by ELISA. The serum content of 5-HT and its related metabolites including kynurenine (KYN), tryptophan (TRP), 5-hydroxytryptophan (5-HTP), and 5-hydroxyindoleacetic acid (5-HIAA) were examined by liquid chromatography-mass spectrometry (LC-MS). The mRNA levels of tryptophan hydroxylase-1 (TPH1), indolamine-2, 3-dioxygenase 1 (IDO1), monoamine oxidase A (MAO-A), 5-hydroxytryptamine 1A receptor (HTR1A), 5-hydroxytryptamine 3 receptor (HTR3), 5-hydroxytryptamine 4 receptor (HTR4) and serotonin reuptake transporter (SERT) were determined by real-time quantitative PCR. Results OVA sensitization caused severe allergic diarrhea in mice. Serum OVA-SIgE increased significantly in mice sensitized by OVA. serum KYN increased remarkably, while 5-HT, 5-HIAA and 5-HTP decreased significantly. The mRNA levels of IDO1, HTR1A and HTR3A increased in gastric tissues, while the levels of TPH1 and MAO-A mRNA decreased. Compared with the model group, the sodium chromate group had lowed systemic score, faeces score, body temperature and OVA-SIgE as well as diarrhea rate. The mRNA levels of 5-HIAA and MAO-A increased in the gastric tissues, and IDO1, 5-HT1A and 5-HT3A mRNAs decreased in the sodium chromate group. Conclusion The serotonin signaling system in ovalbumin-sensitized allergic diarrhea mice has been activated. The administration of sodium chromate can alleviate the allergic symptoms, and change the levels of serum metabolites and the gene expressions of the 5-HT metabolic pathway and its receptors in the stomach.
An Oxidative Bioconjugation Strategy Targeted to a Genetically Encoded 5-Hydroxytryptophan.[Pubmed:29644794]
Chembiochem. 2018 Jul 4;19(13):1375-1378.
Approaches that enable the chemoselective, covalent modification of proteins in a site-specific manner have emerged as a powerful technology for a wide range of applications. The electron-rich unnatural amino acid 5-hydroxytryptophan was recently genetically encoded in both Escherichia coli and eukaryotes, thereby allowing its site-specific incorporation into virtually any recombinant protein. Herein, we report the chemoselective conjugation of various aromatic amines to full-length proteins under mild, oxidative conditions that target this site-specifically incorporated 5-hydroxytryptophan residue.
Metabolic pathway engineering for high-level production of 5-hydroxytryptophan in Escherichia coli.[Pubmed:29933064]
Metab Eng. 2018 Jul;48:279-287.
Cellular metabolic networks should be carefully balanced using metabolic engineering to produce the desired products at the industrial scale. As the precursor for the biosynthesis of the neurotransmitter serotonin, 5-hydroxytryptophan (5-HTP) is effective in treating a variety of diseases, such as depression, fibromyalgia, obesity, and cerebellar ataxia. Due to the lack of an efficient synthetic method, commercial production of 5-HTP is only achieved by extracting from the seeds of Griffonia Smplicifolia. This study reports efficient microbial production of 5-HTP via metabolically engineered Escherichia coli. Firstly, human tryptophan hydroxylase I (TPH1) gene was functionally expressed. For endogenous supply of the cofactor tetrahydrobiopterin (BH4), human BH4 biosynthesis and regeneration pathway was reconstituted. Whole-cell bioconversion resulted in high-level production of 5-HTP (~1.2g/L) from 2g/L L-tryptophan in shake flasks. Further metabolic engineering efforts were employed to achieve 5-HTP biosynthesis from simple carbon sources. The whole biosynthetic pathway was divided into three functional modules, L-tryptophan module, the hydroxylation module, and the BH4 module. By reducing the copy number of L-tryptophan module, replacing TPH1 with a more stable mutant form, and promoter regulation of the BH4 module, 5-HTP was produced at a final titer of 1.3g/L in the shake flask and 5.1g/L in a fed-batch fermenter with glycerol as the carbon source, both of which were the highest ever reported for microbial production of 5-HTP.


