5-MethoxyseselinCAS# 31525-76-5 |
Quality Control & MSDS
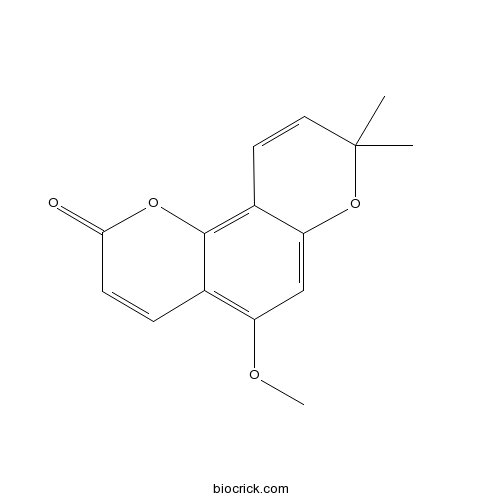
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31525-76-5 | SDF | Download SDF |
| PubChem ID | 290897 | Appearance | Powder |
| Formula | C15H14O4 | M.Wt | 258.27 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-methoxy-8,8-dimethylpyrano[2,3-f]chromen-2-one | ||
| SMILES | CC1(C=CC2=C3C(=C(C=C2O1)OC)C=CC(=O)O3)C | ||
| Standard InChIKey | ZNMRQYJUVCXNGK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14O4/c1-15(2)7-6-10-12(19-15)8-11(17-3)9-4-5-13(16)18-14(9)10/h4-8H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-Methoxyseselin Dilution Calculator

5-Methoxyseselin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8719 mL | 19.3596 mL | 38.7192 mL | 77.4383 mL | 96.7979 mL |
| 5 mM | 0.7744 mL | 3.8719 mL | 7.7438 mL | 15.4877 mL | 19.3596 mL |
| 10 mM | 0.3872 mL | 1.936 mL | 3.8719 mL | 7.7438 mL | 9.6798 mL |
| 50 mM | 0.0774 mL | 0.3872 mL | 0.7744 mL | 1.5488 mL | 1.936 mL |
| 100 mM | 0.0387 mL | 0.1936 mL | 0.3872 mL | 0.7744 mL | 0.968 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Seselin
Catalog No.:BCN9450
CAS No.:523-59-1
- 12-O-Methylinophyllum A
Catalog No.:BCN9449
CAS No.:2131757-10-1
- (-)-Toddalolactone 3′-O-β-D-glucopyranoside
Catalog No.:BCN9448
CAS No.:1176645-57-0
- ent-15α-Acetoxy-11α-hydroxykaur-16-en-19-oic acid
Catalog No.:BCN9447
CAS No.:70324-38-8
- Uncarine F
Catalog No.:BCN9446
CAS No.:14019-66-0
- Angustine
Catalog No.:BCN9445
CAS No.:40041-96-1
- Caloxanthone B
Catalog No.:BCN9444
CAS No.:155233-17-3
- Stipuleanoside R1
Catalog No.:BCN9443
CAS No.:96627-79-1
- Adenostemmoic acid C
Catalog No.:BCN9442
CAS No.:130217-18-4
- Withaphysalin C
Catalog No.:BCN9441
CAS No.:57485-60-6
- ent-Toddalolactone
Catalog No.:BCN9440
CAS No.:1570054-19-1
- 1-Oxohederagenin
Catalog No.:BCN9439
CAS No.:618390-67-3
- 3,6-Dihydroxy-1,2,7-trimethoxyxanthone
Catalog No.:BCN9452
CAS No.:916210-79-2
- Trigochinin A
Catalog No.:BCN9453
CAS No.:1210299-29-8
- Isozedoarondiol
Catalog No.:BCN9454
CAS No.:108887-68-9
- 1,8-Dihydroxy-p-menth-3-en-2-one
Catalog No.:BCN9455
CAS No.:1392224-56-4
- Toddalolactone 3′-O-ethyl ether
Catalog No.:BCN9456
CAS No.:1538607-30-5
- Schisandrolic acid
Catalog No.:BCN9457
CAS No.:55511-17-6
- Toddalosin ethyl ether
Catalog No.:BCN9458
CAS No.:1538607-31-6
- Toddalolactone 3′-O-methyl ether
Catalog No.:BCN9459
CAS No.:143614-35-1
- Phrymarolin B
Catalog No.:BCN9460
CAS No.:1363160-29-5
- Corialin B
Catalog No.:BCN9461
CAS No.:1325717-47-2
- 4α,8β-Dihydroxy-3α-(2-hydroxy-3-acetoxy-2-methylbutyryloxy)eudesm-7(11)-en-12,8α-olide
Catalog No.:BCN9462
CAS No.:1442989-33-4
- 2',6'-Dimethoxypaulownin
Catalog No.:BCN9463
CAS No.:115196-22-0
A catalyst-free one-pot construction of skeletons of 5-methoxyseselin and alloxanthoxyletin in water.[Pubmed:23869658]
Org Lett. 2013 Aug 2;15(15):3856-9.
In refluxing water and without an additional catalyst, electron-rich phenols could react with alkynoic acids or alkynoates to provide coumarin structures. The skeletons of two natural pyranocoumarins, 5-Methoxyseselin and alloxanthoxyletin, could be constructed (total yield up to 76%) in an aqueous multicomponent reaction in which isoprenyl acetate, propiolic acid, and phloroglucinol were simply mixed and refluxed in water.
Natural and synthetic 2,2-dimethylpyranocoumarins with antibacterial activity.[Pubmed:15679322]
J Nat Prod. 2005 Jan;68(1):78-82.
A new efficient synthetic approach to the natural coumarins 5-hydroxyseselin (5), 5-Methoxyseselin (3), and (+/-) cis-grandmarin (9) is described as well as the synthesis of some new derivatives in the 5-Methoxyseselin series (10-15). The natural coumarins 7-hydroxyalloxanthyletin (6), alloxanthoxyletin (8), and dipetalolactone (7) have also been obtained as secondary products. The type of fusion of the pyrano ring in all cases has been established by 2D NMR spectroscopy. The compounds have been studied for their in vitro antibacterial activity, which has been compared with that of some previously synthesized seselin derivatives. The most active compounds were 3, 7, 8, 11, and 14. Some structure-activity relationships are discussed.
Cytotoxic naphthoquinones and plumbagic acid glucosides from Plumbago zeylanica.[Pubmed:12560036]
Phytochemistry. 2003 Feb;62(4):619-22.
Two plumbagic acid glucosides, 3'-O-beta-glucopyranosyl plumbagic acid and 3'-O-beta-glucopyranosyl plumbagic acid methylester along with five naphthoquinones (plumbagin, chitranone, maritinone, elliptinone and isoshinanolone), and five coumarins (seselin, 5-Methoxyseselin, suberosin, xanthyletin and xanthoxyletin) were isolated from the roots of Plumbago zeylanica. All coumarins were not previously found in this plant. Cytotoxicity of these compounds to various tumor cells lines was evaluated, and plumbagin significantly suppressed growth of Raji, Calu-1, HeLa, and Wish tumor cell lines.


