8-DemethyleucalyptinCAS# 5689-38-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5689-38-3 | SDF | Download SDF |
| PubChem ID | 15715157 | Appearance | Powder |
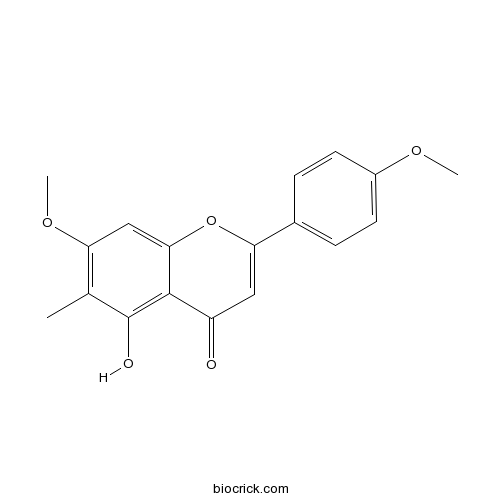
| Formula | C18H16O5 | M.Wt | 312.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-7-methoxy-2-(4-methoxyphenyl)-6-methylchromen-4-one | ||
| SMILES | CC1=C(C=C2C(=C1O)C(=O)C=C(O2)C3=CC=C(C=C3)OC)OC | ||
| Standard InChIKey | QPWOSZAYIILLKU-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | C-methylflavonoids isolated from Callistemon lanceolatus protect PC12 cells against Abeta-induced toxicity.[Pubmed: 20101562]Planta Med. 2010 Jun;76(9):863-8.
|

8-Demethyleucalyptin Dilution Calculator

8-Demethyleucalyptin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.202 mL | 16.0102 mL | 32.0205 mL | 64.041 mL | 80.0512 mL |
| 5 mM | 0.6404 mL | 3.202 mL | 6.4041 mL | 12.8082 mL | 16.0102 mL |
| 10 mM | 0.3202 mL | 1.601 mL | 3.202 mL | 6.4041 mL | 8.0051 mL |
| 50 mM | 0.064 mL | 0.3202 mL | 0.6404 mL | 1.2808 mL | 1.601 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3202 mL | 0.6404 mL | 0.8005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hemopressin (rat)
Catalog No.:BCC5807
CAS No.:568588-77-2
- Z-Glu(OBzl)-OH
Catalog No.:BCC2777
CAS No.:5680-86-4
- H-Ser-OMe.HCl
Catalog No.:BCC3029
CAS No.:5680-80-8
- H-Gly-OMe.HCl
Catalog No.:BCC2951
CAS No.:5680-79-5
- Tanshinone I
Catalog No.:BCN5764
CAS No.:568-73-0
- Tanshinone IIA
Catalog No.:BCN5763
CAS No.:568-72-9
- Melicopine
Catalog No.:BCC8210
CAS No.:568-01-4
- Lycoramine
Catalog No.:BCN2866
CAS No.:21133-52-8
- Scopoletin acetate
Catalog No.:BCN5762
CAS No.:56795-51-8
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Phenylalanine betaine
Catalog No.:BCN5761
CAS No.:56755-22-7
- 1-Methoxyallocryptopine
Catalog No.:BCN7454
CAS No.:56743-52-3
- Carcinine ditrifluoroacetate
Catalog No.:BCC7291
CAS No.:56897-53-1
- Chlorotrianisene
Catalog No.:BCC6442
CAS No.:569-57-3
- Penduletin
Catalog No.:BCN5767
CAS No.:569-80-2
- Xanthohumol
Catalog No.:BCN5768
CAS No.:569-83-5
- Nepetin-7-glucoside
Catalog No.:BCN2580
CAS No.:569-90-4
- Rhamnocitrin
Catalog No.:BCN4619
CAS No.:569-92-6
- Splitomicin
Catalog No.:BCC3652
CAS No.:5690-03-9
- Boc-Ser(Tos)-OMe
Catalog No.:BCC3446
CAS No.:56926-94-4
- UBP 301
Catalog No.:BCC7172
CAS No.:569371-10-4
- 2'-O-Galloylmyricitrin
Catalog No.:BCN8252
CAS No.:56939-52-7
- Withanolide B
Catalog No.:BCN8011
CAS No.:56973-41-2
- Alnusdiol
Catalog No.:BCN6503
CAS No.:56973-51-4
C-methylflavonoids isolated from Callistemon lanceolatus protect PC12 cells against Abeta-induced toxicity.[Pubmed:20101562]
Planta Med. 2010 Jun;76(9):863-8.
Increased beta-amyloid (Abeta) production and its aggregation to the oligomeric state is considered to be a major cause of Alzheimer's disease (AD). Therefore, reducing Abeta-induced neurotoxicity could provide a suitable means of prevention or intervention in the disease course of AD. The neuroprotective effects of isolates from Callistemon lanceolatus DC. (Myrtaceae) against Abeta were evaluated using PC12 cells. To evaluate the effects of Abeta on apoptotic cell death and the effects of Bcl-2 family proteins and caspase-3, TUNEL assays and Western blotting were performed, respectively. Substantial fractionation and purification of the EtOAc-soluble extract of the aerial parts of C. lanceolatus afforded six flavonoids, 4',5-dihydroxy-6,8-dimethyl-7-methoxyflavanone (1), eucalyptin (2), 8-Demethyleucalyptin (3), sideroxylin (4), syzalterin (5), and quercetin (6). Compounds 1, 5, and 6 were found to protect PC12 cells effectively against Abeta-induced toxicity. In particular, compound 1 showed the most promising neuroprotective effect with an ED (50) value of 6.7 microM in terms of decreasing Abeta-induced apoptotic cell death, and this was accompanied by a decrease in caspase-3 activation and an increase in Bcl-2/Bax ratio. These results suggest that compound 1 could be developed as a candidate anti-AD agent due to its attenuation of Abeta-induced apoptotic cell death.


