PenduletinCAS# 569-80-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 569-80-2 | SDF | Download SDF |
| PubChem ID | 5320462 | Appearance | Yellow powder |
| Formula | C18H16O7 | M.Wt | 344.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 4',5-Dihydroxy 3,6,7-trimethoxyflavone; 6-Hydroxykaempferol 3,6,7-trimethyl ether | ||
| Solubility | Soluble in methan | ||
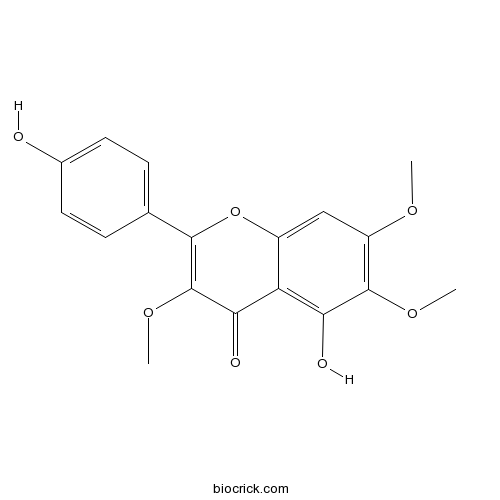
| Chemical Name | 5-hydroxy-2-(4-hydroxyphenyl)-3,6,7-trimethoxychromen-4-one | ||
| SMILES | COC1=C(C(=C2C(=C1)OC(=C(C2=O)OC)C3=CC=C(C=C3)O)O)OC | ||
| Standard InChIKey | YSXFFLGRZJWNFM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O7/c1-22-12-8-11-13(14(20)17(12)23-2)15(21)18(24-3)16(25-11)9-4-6-10(19)7-5-9/h4-8,19-20H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Penduletin shows anti- tumor cells activity. 2. Penduletin significantly reduces TGF-β1 production. 3. Penduletin has strong activity in vitro against EV71 with low cytotoxicity. 4. Penduletin inhibits growth of the Gram-negative pathogen neisseria gonorrhoeae. 5. Penduletin may have anti-inflammatory activity, it can partially inhibit synovial phospholipase A2 activity. |
| Targets | VEGFR | TGF-β/Smad | PGE | COX | Antifection |

Penduletin Dilution Calculator

Penduletin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9044 mL | 14.5222 mL | 29.0444 mL | 58.0889 mL | 72.6111 mL |
| 5 mM | 0.5809 mL | 2.9044 mL | 5.8089 mL | 11.6178 mL | 14.5222 mL |
| 10 mM | 0.2904 mL | 1.4522 mL | 2.9044 mL | 5.8089 mL | 7.2611 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5809 mL | 1.1618 mL | 1.4522 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5809 mL | 0.7261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chlorotrianisene
Catalog No.:BCC6442
CAS No.:569-57-3
- Carcinine ditrifluoroacetate
Catalog No.:BCC7291
CAS No.:56897-53-1
- 8-Demethyleucalyptin
Catalog No.:BCN5765
CAS No.:5689-38-3
- Hemopressin (rat)
Catalog No.:BCC5807
CAS No.:568588-77-2
- Z-Glu(OBzl)-OH
Catalog No.:BCC2777
CAS No.:5680-86-4
- H-Ser-OMe.HCl
Catalog No.:BCC3029
CAS No.:5680-80-8
- H-Gly-OMe.HCl
Catalog No.:BCC2951
CAS No.:5680-79-5
- Tanshinone I
Catalog No.:BCN5764
CAS No.:568-73-0
- Tanshinone IIA
Catalog No.:BCN5763
CAS No.:568-72-9
- Melicopine
Catalog No.:BCC8210
CAS No.:568-01-4
- Lycoramine
Catalog No.:BCN2866
CAS No.:21133-52-8
- Scopoletin acetate
Catalog No.:BCN5762
CAS No.:56795-51-8
- Xanthohumol
Catalog No.:BCN5768
CAS No.:569-83-5
- Nepetin-7-glucoside
Catalog No.:BCN2580
CAS No.:569-90-4
- Rhamnocitrin
Catalog No.:BCN4619
CAS No.:569-92-6
- Splitomicin
Catalog No.:BCC3652
CAS No.:5690-03-9
- Boc-Ser(Tos)-OMe
Catalog No.:BCC3446
CAS No.:56926-94-4
- UBP 301
Catalog No.:BCC7172
CAS No.:569371-10-4
- 2'-O-Galloylmyricitrin
Catalog No.:BCN8252
CAS No.:56939-52-7
- Withanolide B
Catalog No.:BCN8011
CAS No.:56973-41-2
- Alnusdiol
Catalog No.:BCN6503
CAS No.:56973-51-4
- Platyphyllenone
Catalog No.:BCN5766
CAS No.:56973-65-0
- 9,9'-Di-O-(E)-feruloylsecoisolariciresinol
Catalog No.:BCN1415
CAS No.:56973-66-1
- Gabexate mesylate
Catalog No.:BCC2096
CAS No.:56974-61-9
Inhibition of enterovirus 71 replication by chrysosplenetin and penduletin.[Pubmed:21914477]
Eur J Pharm Sci. 2011 Oct 9;44(3):392-8.
In recent years, enterovirus 71 (EV71) infections have caused an increasing epidemic in young children, accompanying with more severe nervous system disease and more deaths. Unfortunately, there is no specific medication for it so far. Here we investigated the anti-EV71 activity of chrysosplenetin and Penduletin, two o-methylated flavonols isolated from the leaves of Laggera pterodonta. These two compounds were found to have strong activity in vitro against EV71 with low cytotoxicity. In the cytopathic effect (CPE) inhibition assays, both plaque reduction assay and virus yield inhibition assay, the compounds showed a similar 50% inhibitory concentration (IC(50)) value of about 0.20 muM. The selectivity indices (SI) of chrysosplenetin and Penduletin were 107.5 and 655.6 in African green monkey kidney (Vero) cells, and 69.5 and 200.5 in human rhabdomyosarcoma (RD) cells, accordingly. The preliminary mechanism analysis indicates that they function not through blocking virus entry or inactivating virus directly but inhibiting viral RNA replication. In the time-of-addition assay, both compounds inhibited progeny virus production and RNA replication by nearly 100% when introduced within 4h post infection. In addition to EV71, both compounds inhibited several other human enteroviruses with similar efficacy. These findings provide a significant lead for the discovery of anti-EV71 drug.
Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats.[Pubmed:22640929]
Phytochemistry. 2013 Jul;91:236-41.
Obesity contributes to increased risk for several chronic diseases including cardiovascular disease and type 2 diabetes. Xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), was tested for efficacy on biomarkers of metabolic syndrome in 4 week old Zucker fa/fa rats, a rodent model of obesity. Rats received daily oral doses of xanthohumol at 0, 1.86, 5.64, and 16.9 mg/kg BW for 6 weeks. All rats were maintained on a high fat (60% kcal) AIN-93G diet for 3 weeks to induce severe obesity followed by a normal AIN-93G (15% kcal fat) diet for the last 3 weeks of the study. Weekly food intake and body weight were recorded. Plasma cholesterol, glucose, insulin, triglyceride, and monocyte chemoattractant protein-1 (MCP-1) levels were assessed using commercial assay kits. Plasma and liver tissue levels of XN and its metabolites were determined by liquid-chromatography tandem mass spectrometry. Plasma and liver tissue levels of xanthohumol were similar between low and medium dose groups and significantly (p<0.05) elevated in the highest dose group. There was a dose-dependent effect on body weight and plasma glucose levels. The highest dose group (n=6) had significantly lower plasma glucose levels compared to the control group (n=6) in male but not female rats. There was also a significant decrease in body weight for male rats in the highest dose group (16.9 mg/kg BW) compared to rats that received no xanthohumol, which was also not seen for female rats. Plasma cholesterol, insulin, triglycerides, and MCP-1 as well as food intake were not affected by treatment. The findings suggest that xanthohumol has beneficial effects on markers of metabolic syndrome.
Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells.[Pubmed:21093515]
Neurochem Int. 2011 Feb;58(2):153-60.
Xanthohumol (2',4',4-trihydroxy-6'-methoxy-3'-prenylchalcone) is a major chalcone derivative isolated from hop (Humulus lupulus L.) commonly used in brewing due to its bitter flavors. Xanthohumol has anti-carcinogenic, free radical-scavenging, and anti-inflammatory activities, but its precise mechanisms are not clarified yet. The basic leucine zipper (bZIP) protein NRF2 is a key transcription factor mediating the antioxidant and anti-inflammatory responses in animals. Therefore, we tested whether xanthohumol exerts anti-inflammatory activity in mouse microglial BV2 cells via NRF2 signaling. Xanthohumol significantly inhibited the excessive production of inflammatory mediators NO, IL-1beta, and TNF-alpha, and the activation of NF-kappaB signaling in LPS-induced stimulated BV2 cells. Xanthohumol up-regulated the transcription of NAD(P)H:quinone oxidoreductase 1 (NQO1) and heme oxygenase-1 (HO-1), and increased the level of the endogenous antioxidant GSH. In addition, xanthohumol induced nuclear translocation of NRF2 and further activation of ARE promoter-related transcription. The anti-inflammatory response of xanthohumol was attenuated by transfection with NRF2 siRNA and in the presence of the HO-1 inhibitor, ZnPP, but not the NQO1 inhibitor, dicoumarol. Taken together, our study suggests that xanthohumol exerts anti-inflammatory activity through NRF2-ARE signaling and up-regulation of downstream HO-1, and could be an attractive candidate for the regulation of inflammatory responses in the brain.
Antineoplastic agents 540. The Indian Gynandropsis gynandra (Capparidaceae).[Pubmed:16119003]
Oncol Res. 2005;15(2):59-68.
The CH3OH-CH2Cl2 extract of an Indian collection (entire plant) of Gynandropsis gynandra (L.) Briq. was separated based on bioassay results employing cancer cell lines. Six cancer cell growth inhibitors were isolated and found to be known flavone apegenin (4) and flavonols 1-3, 5, and 6. The structure of flavonol 2 was confirmed by X-ray crystal structure determination. All of the five flavonols (1-3, 5, 6) inhibited the murine P388 lymphocytic leukemia cell line with ED50 values of 3.0, 9.2, 4.0, 0.37, and 3.9 microg/ml, respectively. All six of the flavonoids (1-6) also exhibited activity against a panel of six human cancer cell lines. Penduletin (3) inhibited growth of the Gram-negative pathogen Neisseria gonorrhoeae and apegenin (4) inhibited growth of the Gram-positive opportunist Enterococcus faecalis.
Antiinflammatory and lipoxygenase inhibitory compounds from Vitex agnus-castus.[Pubmed:19173281]
Phytother Res. 2009 Sep;23(9):1336-9.
Several secondary metabolites, artemetin (1), casticin (2), 3,3'-dihydroxy-5,6,7,4'-tetramethoxy flavon (3), Penduletin (4), methyl 4-hydroxybenzoate (5), p-hydroxybenzoic acid (6), methyl 3,4-dihydroxybenzoate (7), 5-hydroxy-2-methoxybenzoic acid (8), vanillic acid (9) and 3,4-dihydroxybenzoic acid (10) were isolated from a folkloric medicinal plant, Vitex agnus-castus. The structures of compounds 1-10 were identified with the help of spectroscopic techniques. Compounds 3-10 were isolated for the first time from this plant. These compounds were screened for their antiinflammatory and lipoxygenase inhibitory activities. Compounds 6, 7 and 10 were found to have significant antiinflammatory activity in a cell-based contemporary assay, whereas compounds 1 and 2 exhibited a potent lipoxygenase inhibition.
Flavonoids inhibit angiogenic cytokine production by human glioma cells.[Pubmed:21170924]
Phytother Res. 2011 Jun;25(6):916-21.
VEGF and TGF-beta1 are cytokines that stimulate tissue invasion and angiogenesis. These factors are considered as molecular targets for the therapy of glioblastoma. Bevacizumab, a recombinant humanized monoclonal antibody developed against VEGF, inhibits endothelial cell proliferation and vessel formation. Flavonoids obtained from Dimorphandra mollis and Croton betulaster have been described as proliferation inhibitors of a human glioblastoma derived cell line. VEGF and TGF-beta1 levels were dosed by ELISA in a GL-15 cell line treated with bevacizumab and also with the flavonoids rutin, 5-hydroxy-7,4'-dimethoxyflavone, casticin, apigenin and Penduletin. Rutin reduced the VEGF and TGF-beta1 levels after 24 h but not after 72 h. The other flavonoids significantly reduced TGF-beta1 production. Bevacizumab reduced only the VEGF levels in the supernatant from GL-15 cultures. These results suggest that the flavonoids studied, and commonly used in popular medicine, present an interesting subject of study due to their potential effect as angiogenic factor inhibitors.


