Gabexate mesylateTrypsin-like serine proteinases inhibitor CAS# 56974-61-9 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 56974-61-9 | SDF | Download SDF |
| PubChem ID | 6604561 | Appearance | Powder |
| Formula | C17H27N3O7S | M.Wt | 417.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | FOY | ||
| Solubility | DMSO : ≥ 100 mg/mL (239.53 mM) H2O : 7.14 mg/mL (17.10 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
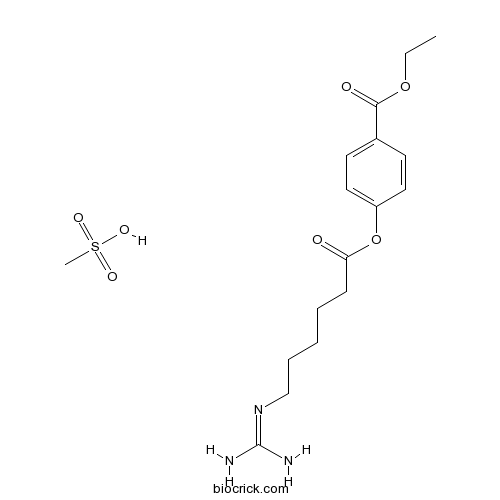
| Chemical Name | ethyl 4-[6-(diaminomethylideneamino)hexanoyloxy]benzoate;methanesulfonic acid | ||
| SMILES | CCOC(=O)C1=CC=C(C=C1)OC(=O)CCCCCN=C(N)N.CS(=O)(=O)O | ||
| Standard InChIKey | DNTNDFLIKUKKOC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H23N3O4.CH4O3S/c1-2-22-15(21)12-7-9-13(10-8-12)23-14(20)6-4-3-5-11-19-16(17)18;1-5(2,3)4/h7-10H,2-6,11H2,1H3,(H4,17,18,19);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Serine protease inhibitor; inhibits trypsin, plasmin, plasma kallikrein and thrombin (IC50 values are 9.4, 30, 41 and 110 μM respectively). Antithrombotic in vitro and in vivo. Also inhibits LPS-induced TNF-α production, probably by inhibiting NF-κB and AP-1 activation. |

Gabexate mesylate Dilution Calculator

Gabexate mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3953 mL | 11.9766 mL | 23.9532 mL | 47.9065 mL | 59.8831 mL |
| 5 mM | 0.4791 mL | 2.3953 mL | 4.7906 mL | 9.5813 mL | 11.9766 mL |
| 10 mM | 0.2395 mL | 1.1977 mL | 2.3953 mL | 4.7906 mL | 5.9883 mL |
| 50 mM | 0.0479 mL | 0.2395 mL | 0.4791 mL | 0.9581 mL | 1.1977 mL |
| 100 mM | 0.024 mL | 0.1198 mL | 0.2395 mL | 0.4791 mL | 0.5988 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gabexate mesylate is a non-antigenic synthetic inhibitor of trypsin-like serine proteinases with Ki values of 3.4nM and 180nM, respectively for human tryptase and bovine tryptase [1].
Gabexate mesylate is a drug used in the treatment of pancreatitis, disseminated intravascular coagulation and as a regional anticoagulant for hemodialysis. Gabexate mesylate is reported to bind to the primary specificity site S1 of human and bovine tryptases. The carbonyl group of gabexate mesylate interacts with the oxyanion binding hole of the enzyme. The tighter binding of gabexate mesylate to human tryptase results in a higher affinity than to bovine tryptase [1].
Gabexate mesylate is also found to inhibit cNOS and iNOS, with Ki values of 150μM and 5mM. It up-regulates iNOS gene expression. In addition, gabexate mesylate inhibits the LPS/IFNγ-induced NO production with EC50 value of 250μM [2].
References:
[1] Erba F, Fiorucci L, Pascarella S, Menegatti E, Ascenzi P, Ascoli F. Selective inhibition of human mast cell tryptase by gabexate mesylate, an antiproteinase drug. Biochem Pharmacol. 2001 Feb 1;61(3):271-6.
[2] Colasanti M, Persichini T, Venturini G, Menegatti E, Lauro GM, Ascenzi P. Effect of gabexate mesylate (FOY), a drug for serine proteinase-mediated diseases, on the nitric oxide pathway. Biochem Biophys Res Commun. 1998 May 19;246(2):453-6.
- 9,9'-Di-O-(E)-feruloylsecoisolariciresinol
Catalog No.:BCN1415
CAS No.:56973-66-1
- Platyphyllenone
Catalog No.:BCN5766
CAS No.:56973-65-0
- Alnusdiol
Catalog No.:BCN6503
CAS No.:56973-51-4
- Withanolide B
Catalog No.:BCN8011
CAS No.:56973-41-2
- 2'-O-Galloylmyricitrin
Catalog No.:BCN8252
CAS No.:56939-52-7
- UBP 301
Catalog No.:BCC7172
CAS No.:569371-10-4
- Boc-Ser(Tos)-OMe
Catalog No.:BCC3446
CAS No.:56926-94-4
- Splitomicin
Catalog No.:BCC3652
CAS No.:5690-03-9
- Rhamnocitrin
Catalog No.:BCN4619
CAS No.:569-92-6
- Nepetin-7-glucoside
Catalog No.:BCN2580
CAS No.:569-90-4
- Xanthohumol
Catalog No.:BCN5768
CAS No.:569-83-5
- Penduletin
Catalog No.:BCN5767
CAS No.:569-80-2
- Boc-Cys(tBu)-OH
Catalog No.:BCC3379
CAS No.:56976-06-8
- U 46619
Catalog No.:BCC7207
CAS No.:56985-40-1
- NSC 87877
Catalog No.:BCC2468
CAS No.:56990-57-9
- Flupirtine
Catalog No.:BCC4282
CAS No.:56995-20-1
- Palmitic acid
Catalog No.:BCN1206
CAS No.:57-10-3
- Stearic Acid
Catalog No.:BCN3820
CAS No.:57-11-4
- Urea
Catalog No.:BCC8034
CAS No.:57-13-6
- Vincristine
Catalog No.:BCN5411
CAS No.:57-22-7
- Strychnine
Catalog No.:BCN4978
CAS No.:57-24-9
- Phenobarbital sodium salt
Catalog No.:BCC6230
CAS No.:57-30-7
- Pentobarbital sodium salt
Catalog No.:BCC6231
CAS No.:57-33-0
- Benactyzine hydrochloride
Catalog No.:BCC8841
CAS No.:57-37-4
Combined derivatization and high-performance liquid chromatography with fluorescence and ultraviolet detection for simultaneous analysis of octreotide and gabexate mesylate metabolite in human pancreatic juice samples.[Pubmed:25354693]
Biomed Chromatogr. 2015 Jun;29(6):911-7.
A simple and sensitive method based on the combination of derivatization and high-performance liquid chromatography with ultraviolet and fluorimetric detection was developed for the simultaneous determination of octreotide and Gabexate mesylate metabolite in human pancreatic juice samples. Parameters of the derivatization procedure affecting extraction efficiency were optimized. The developed method was validated according to the International Conference on Harmonization guidelines. The calibration curves were linear over a range of 0.1-15 microg/mL for octreotide and 0.20-15 microg/mL for Gabexate mesylate metabolite. Derivatized products of octreotide and Gabexate mesylate metabolite were separated on a Luna C18 column (4.6 x 250 mm; 5 microm particle size) using a gradient with a run time of 36 min, without further purification. The limits of detection were 0.025 and 0.05, respectively, for octreotide and Gabexate mesylate metabolite. This paper reports the validation of a quantitative high performance liquid chromatography-photodiode array-fluorescence (HPLC-PDA-FL) method for the simultaneous analysis of octreotide and Gabexate mesylate metabolite in pancreatic juice by protein precipitation using zinc sulfate-methanol-acetonitrile containing the derivatizing reagent, 4-fluoro-7-nitro-[2,1,3]-benzoxadiazole (NBD-F). Derivatized products of octreotide and Gabexate mesylate metabolite were separated on a Luna C18 column (4.6 x 250 mm; 5 microm particle size) using a gradient with a run time of 36 min, without further purification. The method was validated over the concentration ranges 0.1-15 and 0.2-15 microg/mL for octreotide and Gabexate mesylate metabolite, respectively, in human pancreatic juice. Biphalin and methyl-p-hydroxybenzoate were used as the internal standards. This method was successfully utilized to support clinical studies in humans. The results from assay validations show that the method is selective, sensitive and robust. The limit of quantification of the method was 0.1 microg/mL for octreotide and 0.2 microg/mL for Gabexate mesylate metabolite, and matrix matched standard curves showed a good linearity up to 15 microg/mL. In the entire analytical range the intra- and inter-day precision (RSD%) values were respectively Gabexate mesylate metabolite. For both analytes the intra- and inter-day accuracy (bias) values ranged respectively from -6.8 to -2.5% and from -4.6 to -5.7%. This method utilizes derivatization with NBD-F and provides adequate sensitivity for both drugs.
Comparison of recombinant human thrombomodulin and gabexate mesylate for treatment of disseminated intravascular coagulation (DIC) with sepsis following emergent gastrointestinal surgery: a retrospective study.[Pubmed:26038004]
Eur J Trauma Emerg Surg. 2015 Oct;41(5):531-8.
PURPOSE: Recombinant thrombomodulin (rTM) has been available in Japan since 2008, but there is concern about its association with postoperative hemorrhage. The efficacy and safety of rTM were examined in patients with disseminated intravascular coagulation (DIC) caused by a septic condition after gastrointestinal surgery. METHODS: Forty-two patients were emergently admitted to the intensive care unit after emergent gastrointestinal surgery in Kyushu University Hospital from May 2008 to April 2013. Of these patients, 22 had DIC (defined as an acute DIC score >/= 4). All but three patients received treatment with Gabexate mesylate (GM) (n = 9) or rTM (n = 10). The causes of sepsis were peritonitis with colorectal perforation, anastomotic leakage, and intestinal necrosis. Acute DIC score, sepsis-related organ failure assessment score, platelet count, and a variety of biochemical parameters were compared between rTM and GM recipients after treatment administration. RESULTS: There were no significant differences between the groups for any parameter except C-reactive protein levels. The CRP level tended to be lower in the rTM group than in the GM group. Acute DIC score in the rTM group resolved significantly earlier than that in the GM group. No patient stopped the administration of rTM because of postoperative bleeding. CONCLUSION: rTM may be an effective therapeutic drug for the treatment of septic patients with DIC following emergent gastrointestinal surgery.
Protection Provided by a Gabexate Mesylate Thermo-Sensitive In Situ Gel for Rats with Grade III Pancreatic Trauma.[Pubmed:27646597]
Gut Liver. 2017 Jan 15;11(1):156-163.
Background/Aims: This study investigated the protection provided by Gabexate mesylate thermo-sensitive in-situ gel (GMTI) against grade III pancreatic trauma in rats. Methods: A grade III pancreatic trauma model with main pancreatic duct dividing was established, and the pancreas anatomical diagram, ascites, and serum biochemical indices, including amylase, lipase, C-reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha), were examined. The pancreas was sliced and stained with hematoxylin eosin and subjected to terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Results: Ascites, serum amylase, lipase, CRP, IL-6, and TNF-alpha levels were significantly increased in the pancreas trauma (PT) groups with prolonged trauma time and were significantly decreased after GMTI treatment. The morphological structure of the pancreas was loose, the acinus was significantly damaged, the nuclei were irregular and hyperchromatic, and there was inflammatory cell invasion in the PT group compared to the control. After GMTI treatment, the morphological structure of the pancreas was restored, and the damaged acinus and inflammatory cell invasion were decreased compared to the PT group. Moreover, the cell apoptosis index was significantly increased in the PT group and restored to the same levels as the control group after GMTI treatment. Conclusions: GMTI, a novel formulation and drug delivery method, exhibited specific effective protection against PT with acute pancreatitis therapy and has potential value as a minimally invasive adjuvant therapy for PT with acute pancreatitis.
Gabexate mesylate as treatment in the course of ANCA-negative microscopic polyangiitis.[Pubmed:23560992]
Ren Fail. 2013;35(5):721-4.
Patients with small vessel vasculitis present fluctuating antineutrophil cytoplasmic antibodies (ANCA) levels to the point that positive ANCA may be missed even if only up to 10% of patients with microscopic polyangiitis (MPA) are ANCA-negative. The first-line treatment of MPA is the association of steroids and cyclophosphamide, especially in the presence of a rapidly progressive glomerulonephritis. Plasmapheresis, intravenous immunoglobulins, and tumor necrosis factor inhibitors have been proposed as alternative to standard therapy. Disseminated intravascular coagulation (DIC) is a possible event in the course of small vessel vasculitis. Gabexate mesylate is a protease inhibitor able to suppress endothelial cell injury, and it may be administered to treat DIC related to different diseases. In ANCA-associated vasculitis, cytokines play a key role in promoting endothelial damage. DIC-related thrombocytopenia may be misinterpreted as drug-induced because of the immunosuppressive properties of cyclophosphamide. Two cases of ANCA-positive MPA associated with DIC and treated with gabexate are reported in the literature with improvement of both hematological disorder and renal function. Our patient presented a rapidly progressive glomerulonephritis, and the renal biopsy showed MPA, in the absence of ANCA. After two weeks of steroid treatment, our patient developed a DIC. This case represents the first report of ANCA-negative MPA managed with gabexate, which showed improvement of coagulation disorders and kidney function. In conclusion, the anti-inflammatory properties of gabexate could be helpful in MPA at increased bleeding risk when immunosuppressive treatment is contraindicated, even in ANCA-negative vasculitis.
Gabexate mesilate, a synthetic protease inhibitor, inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production by inhibiting activation of both nuclear factor-kappaB and activator protein-1 in human monocytes.[Pubmed:12649382]
J Pharmacol Exp Ther. 2003 Apr;305(1):298-305.
Gabexate mesilate, a synthetic protease inhibitor, was shown to be effective in treating patients with sepsis-associated disseminated intravascular coagulation in which tumor necrosis factor-alpha (TNF-alpha) plays a critical role. We demonstrated that gabexate mesilate reduced lipopolysaccharide (LPS)-induced tissue injury by inhibiting TNF-alpha production in rats. In the present study, we analyzed the mechanism(s) by which gabexate mesilate inhibits LPS-induced TNF-alpha production in human monocytes in vitro. Gabexate mesilate inhibited the production of TNF-alpha in monocytes stimulated with LPS. Gabexate mesilate inhibited both the binding of nuclear factor-kappaB (NF-kappaB) to target sites and the degradation of inhibitory kappaBalpha. Gabexate mesilate also inhibited both the binding of activator protein-1 (AP-1) to target sites and the activation of mitogen-activated protein kinase pathways. These observations strongly suggest that gabexate mesilate inhibited LPS-induced TNF-alpha production in human monocytes by inhibiting activation of both NF-kappaB and AP-1. Inhibition of TNF-alpha production by gabexate mesilate might explain at least partly its therapeutic effects in animals given LPS and those in patients with sepsis.
Synthetic inhibitors of trypsin, plasmin, kallikrein, thrombin, C1r-, and C1 esterase.[Pubmed:143965]
Biochim Biophys Acta. 1977 Oct 13;484(2):417-22.
p-Carbethoxyphenyl episol-guanidinocaproate and p-(p'-guanidinobenzoyloxy)-phenyl derivatives were prepared, and their inhibitory effects on trypsin, plasmin, plasma kallikrein, thrombin, C1r- and C1 esterase were examined. Among the various inhibitors tested, p-nitrophenyl p'-guanidinobenzoate, N,N-dimethylamino p-(p'-guanidinobenzoyloxy)-benzoyl glycolate and N,N-dimethylamino p-(p'-guanidinobenzoyloxy)-benzilcarbonyloxy glycolate were the most effective inhibitors of trypsin, plasmin, plasma kallikrien and thrombin, and they strongly inhibited the esterolytic activities of C1r- and C1 esterase.


