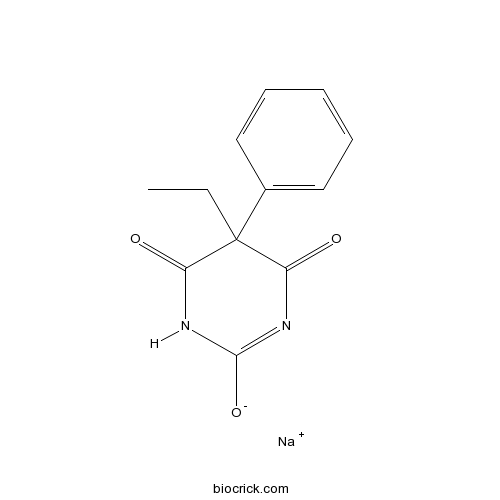
Phenobarbital sodium saltEnhances GABAergic activity CAS# 57-30-7 |
- Metformin HCl
Catalog No.:BCC4799
CAS No.:1115-70-4
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Geniposide
Catalog No.:BCN5104
CAS No.:24512-63-8
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- Imeglimin hydrochloride
Catalog No.:BCC4085
CAS No.:775351-61-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57-30-7 | SDF | Download SDF |
| PubChem ID | 23674889 | Appearance | Powder |
| Formula | C12H11N2NaO3 | M.Wt | 254.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | sodium;5-ethyl-4,6-dioxo-5-phenyl-1H-pyrimidin-2-olate | ||
| SMILES | CCC1(C(=O)NC(=NC1=O)[O-])C2=CC=CC=C2.[Na+] | ||
| Standard InChIKey | WRLGYAWRGXKSKG-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C12H12N2O3.Na/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16;/h3-7H,2H2,1H3,(H2,13,14,15,16,17);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Enhances GABAergic activity. Long-acting barbiturate; slow-acting barbiturate also available. |

Phenobarbital sodium salt Dilution Calculator

Phenobarbital sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9336 mL | 19.668 mL | 39.336 mL | 78.672 mL | 98.34 mL |
| 5 mM | 0.7867 mL | 3.9336 mL | 7.8672 mL | 15.7344 mL | 19.668 mL |
| 10 mM | 0.3934 mL | 1.9668 mL | 3.9336 mL | 7.8672 mL | 9.834 mL |
| 50 mM | 0.0787 mL | 0.3934 mL | 0.7867 mL | 1.5734 mL | 1.9668 mL |
| 100 mM | 0.0393 mL | 0.1967 mL | 0.3934 mL | 0.7867 mL | 0.9834 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Strychnine
Catalog No.:BCN4978
CAS No.:57-24-9
- Vincristine
Catalog No.:BCN5411
CAS No.:57-22-7
- Urea
Catalog No.:BCC8034
CAS No.:57-13-6
- Stearic Acid
Catalog No.:BCN3820
CAS No.:57-11-4
- Palmitic acid
Catalog No.:BCN1206
CAS No.:57-10-3
- Flupirtine
Catalog No.:BCC4282
CAS No.:56995-20-1
- NSC 87877
Catalog No.:BCC2468
CAS No.:56990-57-9
- U 46619
Catalog No.:BCC7207
CAS No.:56985-40-1
- Boc-Cys(tBu)-OH
Catalog No.:BCC3379
CAS No.:56976-06-8
- Gabexate mesylate
Catalog No.:BCC2096
CAS No.:56974-61-9
- 9,9'-Di-O-(E)-feruloylsecoisolariciresinol
Catalog No.:BCN1415
CAS No.:56973-66-1
- Platyphyllenone
Catalog No.:BCN5766
CAS No.:56973-65-0
- Pentobarbital sodium salt
Catalog No.:BCC6231
CAS No.:57-33-0
- Benactyzine hydrochloride
Catalog No.:BCC8841
CAS No.:57-37-4
- Phenytoin
Catalog No.:BCC5070
CAS No.:57-41-0
- Esromiotin
Catalog No.:BCC8325
CAS No.:57-47-6
- Fructose
Catalog No.:BCN4969
CAS No.:57-48-7
- Sucrose
Catalog No.:BCN5780
CAS No.:57-50-1
- Chlorotetracycline
Catalog No.:BCC8913
CAS No.:57-62-5
- Ethinyl Estradiol
Catalog No.:BCC3777
CAS No.:57-63-6
- Probenecid
Catalog No.:BCC4832
CAS No.:57-66-9
- Sulfaguanidine
Catalog No.:BCC4727
CAS No.:57-67-0
- Sulfamethazine
Catalog No.:BCC4942
CAS No.:57-68-1
- Progesterone
Catalog No.:BCN2198
CAS No.:57-83-0
Molecular approaches for production of pravastatin, a HMG-CoA reductase inhibitor: transcriptional regulation of the cytochrome p450sca gene from Streptomyces carbophilus by ML-236B sodium salt and phenobarbital.[Pubmed:9524240]
Gene. 1998 Mar 27;210(1):109-16.
We have characterized the transcriptional regulation of ML-236B.Na and phenobarbital-inducible cytochrome P450sca-2 (CytP450sca-2) from Streptomyces carbophilus, an industrial pravastatin-producing strain. ML-236B.Na and phenobarbital enhanced the expression of the cytP450sca-2 gene in S. carbophilus. The cytP450sca-2 gene was also ML-236B.Na-inductive in S. lividans. Analysis of various deletion and mutation of the 5'-flanking region of the cytP450sca-2 gene revealed that the 1-kb region was required for ML-236B.Na-dependent CytP450sca-2 induction. We have found a putative ORF in the 5'-flanking region that encodes a protein of 174 amino acid residues containing a helix-turn-helix DNA-binding motif. A gel mobility shift assay showed that the protein was bound by an imperfect palindromic sequence between -46bp and -24bp in the 5'-flanking region, and ML-236B.Na was found to inhibit its binding. These findings suggest that induction of cytP450sca-2 is negatively regulated at the transcriptional level and that the protein encoded by the putative ORF is possibly functional as a repressor of the cytP450sca-2 gene.
Phenobarbital but Not Diazepam Reduces AMPA/kainate Receptor Mediated Currents and Exerts Opposite Actions on Initial Seizures in the Neonatal Rat Hippocampus.[Pubmed:21847371]
Front Cell Neurosci. 2011 Jul 28;5:16.
Diazepam (DZP) and phenobarbital (PB) are extensively used as first and second line drugs to treat acute seizures in neonates and their actions are thought to be mediated by increasing the actions of GABAergic signals. Yet, their efficacy is variable with occasional failure or even aggravation of recurrent seizures questioning whether other mechanisms are not involved in their actions. We have now compared the effects of DZP and PB on ictal-like events (ILEs) in an in vitro model of mirror focus (MF). Using the three-compartment chamber with the two immature hippocampi and their commissural fibers placed in three different compartments, kainate was applied to one hippocampus and PB or DZP to the contralateral one, either after one ILE, or after many recurrent ILEs that produce an epileptogenic MF. We report that in contrast to PB, DZP aggravated propagating ILEs from the start, and did not prevent the formation of MF. PB reduced and DZP increased the network driven giant depolarizing potentials suggesting that PB may exert additional actions that are not mediated by GABA signaling. In keeping with this, PB but not DZP reduced field potentials recorded in the presence of GABA and NMDA receptor antagonists. These effects are mediated by a direct action on AMPA/kainate receptors since PB: (i) reduced AMPA/kainate receptor mediated currents induced by focal applications of glutamate; (ii) reduced the amplitude and the frequency of AMPA but not NMDA receptor mediated miniature excitatory postsynaptic currents (EPSCs); (iii) augmented the number of AMPA receptor mediated EPSCs failures evoked by minimal stimulation. These effects persisted in MF. Therefore, PB exerts its anticonvulsive actions partly by reducing AMPA/kainate receptors mediated EPSCs in addition to the pro-GABA effects. We suggest that PB may have advantage over DZP in the treatment of initial neonatal seizures since the additional reduction of glutamate receptors mediated signals may reduce the severity of neonatal seizures.
Calcium current block by (-)-pentobarbital, phenobarbital, and CHEB but not (+)-pentobarbital in acutely isolated hippocampal CA1 neurons: comparison with effects on GABA-activated Cl- current.[Pubmed:8101867]
J Neurosci. 1993 Aug;13(8):3211-21.
Block of a voltage-activated Ca2+ channel current by phenobarbital (PHB), 5-(2-cyclohexylideneethyl)-5-ethyl barbituric acid (CHEB), and the optical R(-)- and S(+)-enantiomers of pentobarbital (PB) was examined in freshly dissociated adult guinea pig hippocampal CA1 neurons; the effects of the barbiturates on GABA-activated Cl- current were also characterized in the same preparation. (-)-PB, PHB, and CHEB produced a reversible, concentration-dependent block of the peak Ca2+ channel current (3 mM Ba2+ as the charge carrier) evoked by depolarization from -80 to -10 mV (IC50 values, 3.5, 72, and 118 microM, respectively). In contrast, (+)-PB was nearly inactive at concentrations up to 1 mM. The inhibitory action of PHB was decreased at acid pH, indicating that the dissociated (anionic) form of the molecule is the active species. Block by (-)-PB was voltage dependent with the fractional block increasing at positive membrane potentials; calculations according to the method of Woodhull indicated that the (-)-PB blocking site senses approximately 40% of the transmembrane electric field. The time course and voltage dependence of activation of the Ca2+ channel current were unaffected by (-)-PB, PHB, and CHEB. The rate of inactivation was enhanced by (-)-PB and CHEB, with the major effect being acceleration of the slow phase of the biexponential decay of the current. GABA-activated Cl- current was potently enhanced by (-)-PB and PHB (EC50 values, 3.4 and 12 microM), whereas (+)-PB was only weakly active. At concentrations of (-)-PB > 100 microM and PHB > 200-300 microM, Cl- current responses were activated even in the absence of GABA. These results demonstrate that in CA1 hippocampal neurons, PB causes a stereoselective block of a voltage-activated Ca2+ current; PHB is also effective, but at higher concentrations. For (-)-PB, the effect on Ca2+ channel current occurred at similar concentrations as potentiation of GABA responses. In contrast, PHB was more potent as a GABA enhancer than as blocker of Ca2+ current, but the maximal potentiation of GABA responses was 40% of that obtained with (-)-PB. Consequently, the anticonvulsant action of PHB at clinically relevant concentrations may relate to modest enhancement of GABA responses and partial blockade of Ca2+ current, whereas the sedative effects that occur at higher concentrations could reflect stronger Ca2+ current blockade. The powerful sedative-hypnotic action of (-)-PB may reflect greater maximal enhancement of GABA responses in conjunction with strong inhibition of Ca2+ current.(ABSTRACT TRUNCATED AT 400 WORDS)


