Allyl phenoxyacetateCAS# 7493-74-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7493-74-5 | SDF | Download SDF |
| PubChem ID | 24117 | Appearance | Powder |
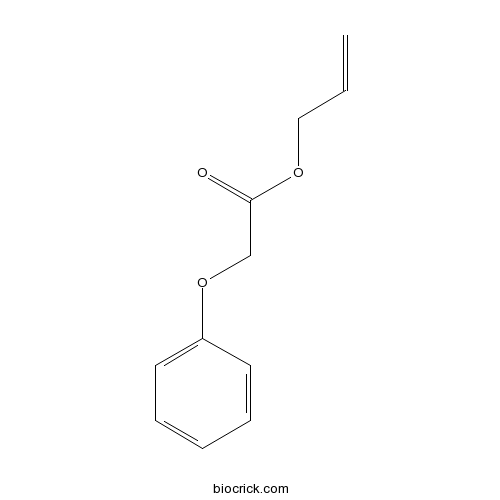
| Formula | C11H12O3 | M.Wt | 192 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | prop-2-enyl 2-phenoxyacetate | ||
| SMILES | C=CCOC(=O)COC1=CC=CC=C1 | ||
| Standard InChIKey | VUFZVGQUAVDKMC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H12O3/c1-2-8-13-11(12)9-14-10-6-4-3-5-7-10/h2-7H,1,8-9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Allyl phenoxyacetate Dilution Calculator

Allyl phenoxyacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2083 mL | 26.0417 mL | 52.0833 mL | 104.1667 mL | 130.2083 mL |
| 5 mM | 1.0417 mL | 5.2083 mL | 10.4167 mL | 20.8333 mL | 26.0417 mL |
| 10 mM | 0.5208 mL | 2.6042 mL | 5.2083 mL | 10.4167 mL | 13.0208 mL |
| 50 mM | 0.1042 mL | 0.5208 mL | 1.0417 mL | 2.0833 mL | 2.6042 mL |
| 100 mM | 0.0521 mL | 0.2604 mL | 0.5208 mL | 1.0417 mL | 1.3021 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DMAT
Catalog No.:BCC1533
CAS No.:749234-11-5
- Piracetam
Catalog No.:BCC4824
CAS No.:7491-74-9
- 5-Acetoxymatairesinol dimethyl ether
Catalog No.:BCN4300
CAS No.:74892-45-8
- 5-Carboxamidotryptamine maleate
Catalog No.:BCC6652
CAS No.:74885-72-6
- Vernakalant Hydrochloride
Catalog No.:BCC2037
CAS No.:748810-28-8
- alpha-Carotene
Catalog No.:BCN3880
CAS No.:7488-99-5
- Argatroban
Catalog No.:BCC3723
CAS No.:74863-84-6
- Boc-Hyp-Ome
Catalog No.:BCC3252
CAS No.:74844-91-0
- GRP (porcine)
Catalog No.:BCC5809
CAS No.:74815-57-9
- 3',5'-Anhydrothymidine
Catalog No.:BCC8596
CAS No.:7481-90-5
- Zalcitabine
Catalog No.:BCC5026
CAS No.:7481-89-2
- Methylophiopogonanone A
Catalog No.:BCN5417
CAS No.:74805-92-8
- NSC 405020
Catalog No.:BCC2120
CAS No.:7497-07-6
- 2-((1,1-Dioxidotetrahydrothiophen-3-yl)(methyl)amino)-2-oxoethyl 3-(3-nitrophenyl)acrylate
Catalog No.:BCC6185
CAS No.:749872-43-3
- JSH-23
Catalog No.:BCC4610
CAS No.:749886-87-1
- Callimorphine
Catalog No.:BCN1959
CAS No.:74991-73-4
- 9-Methoxycanthin-6-one
Catalog No.:BCN2993
CAS No.:74991-91-6
- Ethylamine
Catalog No.:BCN1799
CAS No.:75-04-7
- 1,2-Cyclohexanedicarboximide
Catalog No.:BCC8416
CAS No.:7506-66-3
- Daphnilongeranin C
Catalog No.:BCN4301
CAS No.:750649-07-1
- 20,24-Dihydroxydammar-25-en-3-one
Catalog No.:BCN4302
CAS No.:75069-59-9
- 2',6'-Dihydroxy-4'-methoxyacetophenone
Catalog No.:BCN6891
CAS No.:7507-89-3
- Obacunone
Catalog No.:BCN4303
CAS No.:751-03-1
- 2-Acetamido-2-deoxy-D-glucose
Catalog No.:BCC8508
CAS No.:7512-17-6
In situ synthesis of a monolithic material with multi-sized pores and its chromatographic properties for the separation of intact proteins from human plasma.[Pubmed:30609551]
Talanta. 2019 Mar 1;194:406-414.
The fabrication of a monolithic Allyl phenoxyacetate-based material was proposed via the in situ radical polymerization using ethylene dimethacrylate as the crosslinker and 2,2'-azobisisobutyronitrile as the initiator within a stainless steel column (50mmx4.6mm i.d.). The effects of the porogen composition, the crosslinker amount and the monomer type on the resulting monoliths were investigated. The morphology of the monoliths was characterized using scanning electron microscopy and a nitrogen adsorption-desorption instrument, and the pore structure was characterized using mercury intrusion porosimetry. The results indicate that the optimized monolith has a micro-, meso- and macro- multi-sized pore structure with a high specific surface area of 260.66m(2) g(-1). The resulting monoliths were used as stationary phases for the separation of proteins from bio-samples, including a mixture of six standard proteins, chicken egg whites, snailase and human plasma, using high-performance liquid chromatography. Compared to optimized glycidyl methacrylate-based and styrene-based monolithic columns, the Allyl phenoxyacetate-based monolithic column exhibited improved selectivity in the separation of proteins. Furthermore, the present method avoids the masking of highly abundant proteins, such as human serum albumin, immunoglobulin G and human fibrinogen, in the detection of middle- or low-abundance proteins in human plasma. The protein identification results obtained from liquid chromatography/mass spectrometry indicate that the present method is an outstanding method for efficient fractionation of human plasma, which will be especially useful in future plasma proteomics research.
The effects of vehicles on the human dermal irritation potentials of allyl esters.[Pubmed:16717034]
Int J Toxicol. 2006 May-Jun;25(3):183-93.
Allyl esters, frequently used in the fragrance industry, often contain a certain percentage of free allyl alcohol. Allyl alcohol is known to have a potential for delayed skin irritation. Also present in the finished product are different solvent systems, or vehicles, which are used to deliver the fragrances based upon their intended application. This study was conducted to determine whether different vehicles affect the skin irritation potential of five different allyl esters. The allyl esters tested were allyl amyl glycolate, allyl caproate, allyl (cyclohexyloxy)acetate, allyl cyclohexylpropionate, and Allyl phenoxyacetate in the vehicles diethyl phthalate, 3:1 diethyl phthalate:ethanol, and 1:3 diethyl phthalate:ethanol at concentrations of 0.1%, 0.5%, 1.0%, and 2.0% (w/w). A modified cumulative irritation test was conducted in 129 human subjects. Test materials (0.3 ml) were applied under occlusion to skin sites on the back for 1 day (24 h) using Hill Top chambers. Irritation was assessed at 1, 2, 4, and 5 days following application of test materials. Cumulative irritation scores varied considerably among test materials. There were no delayed irritation observations. The highest irritation scores were observed at the 2.0% concentration for all test materials. The irritation scores for allyl amyl glycolate, allyl (cyclohexyloxy)acetate, and Allyl phenoxyacetate were highest in 1:3 diethyl phthalate:ethanol, thus the resulting calculated no-observed-effect levels, 0.12%, 0.03%, and 0%, respectively, were much lower for this vehicle compared to the diethyl phthalate vehicle, 0.33%, 0.26%, 0.25%, respectively. These data showed a trend for lower concentration thresholds to induce irritation when higher levels of ethanol were used in the vehicle.


