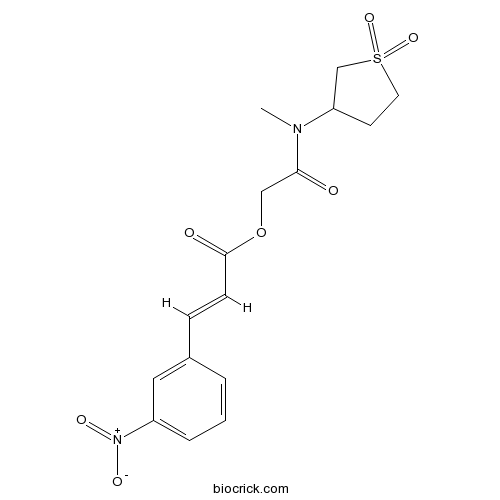
2-((1,1-Dioxidotetrahydrothiophen-3-yl)(methyl)amino)-2-oxoethyl 3-(3-nitrophenyl)acrylateCAS# 749872-43-3 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 749872-43-3 | SDF | Download SDF |
| PubChem ID | 5951923 | Appearance | Powder |
| Formula | C16H18N2O7S | M.Wt | 382.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | [2-[(1,1-dioxothiolan-3-yl)-methylamino]-2-oxoethyl] (E)-3-(3-nitrophenyl)prop-2-enoate | ||
| SMILES | CN(C1CCS(=O)(=O)C1)C(=O)COC(=O)C=CC2=CC(=CC=C2)[N+](=O)[O-] | ||
| Standard InChIKey | URVRJYLSUVXWBC-AATRIKPKSA-N | ||
| Standard InChI | InChI=1S/C16H18N2O7S/c1-17(14-7-8-26(23,24)11-14)15(19)10-25-16(20)6-5-12-3-2-4-13(9-12)18(21)22/h2-6,9,14H,7-8,10-11H2,1H3/b6-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of Krüppel-like factor 5 (KLF5) transcription factor (IC50 = 2.3 μM). Selectively active against colon cancer cells in a panel of 60 different cancer cell lines. |

2-((1,1-Dioxidotetrahydrothiophen-3-yl)(methyl)amino)-2-oxoethyl 3-(3-nitrophenyl)acrylate Dilution Calculator

2-((1,1-Dioxidotetrahydrothiophen-3-yl)(methyl)amino)-2-oxoethyl 3-(3-nitrophenyl)acrylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6151 mL | 13.0757 mL | 26.1513 mL | 52.3026 mL | 65.3783 mL |
| 5 mM | 0.523 mL | 2.6151 mL | 5.2303 mL | 10.4605 mL | 13.0757 mL |
| 10 mM | 0.2615 mL | 1.3076 mL | 2.6151 mL | 5.2303 mL | 6.5378 mL |
| 50 mM | 0.0523 mL | 0.2615 mL | 0.523 mL | 1.0461 mL | 1.3076 mL |
| 100 mM | 0.0262 mL | 0.1308 mL | 0.2615 mL | 0.523 mL | 0.6538 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NSC 405020
Catalog No.:BCC2120
CAS No.:7497-07-6
- Allyl phenoxyacetate
Catalog No.:BCC8813
CAS No.:7493-74-5
- DMAT
Catalog No.:BCC1533
CAS No.:749234-11-5
- Piracetam
Catalog No.:BCC4824
CAS No.:7491-74-9
- 5-Acetoxymatairesinol dimethyl ether
Catalog No.:BCN4300
CAS No.:74892-45-8
- 5-Carboxamidotryptamine maleate
Catalog No.:BCC6652
CAS No.:74885-72-6
- Vernakalant Hydrochloride
Catalog No.:BCC2037
CAS No.:748810-28-8
- alpha-Carotene
Catalog No.:BCN3880
CAS No.:7488-99-5
- Argatroban
Catalog No.:BCC3723
CAS No.:74863-84-6
- Boc-Hyp-Ome
Catalog No.:BCC3252
CAS No.:74844-91-0
- GRP (porcine)
Catalog No.:BCC5809
CAS No.:74815-57-9
- 3',5'-Anhydrothymidine
Catalog No.:BCC8596
CAS No.:7481-90-5
- JSH-23
Catalog No.:BCC4610
CAS No.:749886-87-1
- Callimorphine
Catalog No.:BCN1959
CAS No.:74991-73-4
- 9-Methoxycanthin-6-one
Catalog No.:BCN2993
CAS No.:74991-91-6
- Ethylamine
Catalog No.:BCN1799
CAS No.:75-04-7
- 1,2-Cyclohexanedicarboximide
Catalog No.:BCC8416
CAS No.:7506-66-3
- Daphnilongeranin C
Catalog No.:BCN4301
CAS No.:750649-07-1
- 20,24-Dihydroxydammar-25-en-3-one
Catalog No.:BCN4302
CAS No.:75069-59-9
- 2',6'-Dihydroxy-4'-methoxyacetophenone
Catalog No.:BCN6891
CAS No.:7507-89-3
- Obacunone
Catalog No.:BCN4303
CAS No.:751-03-1
- 2-Acetamido-2-deoxy-D-glucose
Catalog No.:BCC8508
CAS No.:7512-17-6
- PRE-084 hydrochloride
Catalog No.:BCC6708
CAS No.:75136-54-8
- H-Leu-OMe.HCl
Catalog No.:BCC2973
CAS No.:7517-19-3
Profiling of hydroxycinnamoylquinic acids in plant extracts using in-source CID fragmentation.[Pubmed:27591562]
J Mass Spectrom. 2016 Dec;51(12):1130-1145.
Hydroxycinnamoylquinic acids (HCQAs) are a major class of phenolic plant secondary metabolites, belonging to the chlorogenic acid family. Various health-beneficial properties of HCQAs have been shown, which has drawn interest for HCQA profiling in plants of human consumption. However, this task remains challenging, because several isomeric HCQAs can be present in the sample with identical molecular formulae and the limited availability of reference standards poses additional challenges to their identification. In the present work, a high performance liquid chromatography-electrospray ionization-quadrupole time-of-flight-mass spectrometry (HPLC-ESI-Q/TOF-MS) method accompanied with an effective data filtering protocol is presented, which is shown to be suitable for the identification of HCQAs in plant materials in a non-targeted manner. Both collision-induced dissociation (CID) fragmentation performed in a collision cell and in-source (CID) fragmentation were used to produce accurate mass fragments. It was shown that fragmentation characteristics required for identification of regio-isomers of HCQAs can be achieved with in-source CID fragmentation, enabling the use of a single-stage MS system with in-source fragmentation for convincing identification of HCQAs. Based on a thorough validation of identified HCQA compounds using coffee bean extracts as reference samples, comprehensive profiling of HCQAs in two apricot (Prunus armeniaca L.) genotypes ('Preventa' and 'Gonci magyarkajszi') was carried out for the first time and the following 10 HCQAs were shown to be present in apricot fruit: 3-caffeoylquinic acid (CQA), cis-3-CQA, 4-CQA, 5-CQA, cis-5-CQA, 3,5-diCQA, 3-p-coumaroylquinic acid (pCoQA), 4-pCoQA, 3-feruloylquinic acid (FQA) and cis-3-FQA. Copyright (c) 2016 John Wiley & Sons, Ltd. HIGHLIGHTS: An HPLC-ESI-Q/TOF-MS method suitable for the identification of hydroxycinnamoyilquinic acids (HCQAs) in plant material in a non-targeted manner was developed. Single-stage, high-resolution MS system with in-source fragmentation was shown to be suitable for convincing identification of HCQAs. Comprehensive profiling of HCQAs in two apricot (Prunus armeniaca L.) genotypes was carried out for the first time. Copyright (c) 2016 John Wiley & Sons, Ltd.
Analysis of matrix-assisted laser desorption/ionization quadrupole time-of-flight collision-induced dissociation spectra of simple precursor ions and isobaric oligosaccharide ion mixtures based on product ion intensities and pattern recognition.[Pubmed:28299859]
Rapid Commun Mass Spectrom. 2017 May 30;31(10):873-885.
RATIONALE: Qualitative analysis of glycomic tandem mass spectrometry (MS/MS) data based on m/z values of product ions alone is widely used, and often sufficient for analysis of single analytes. However, most complex glycomic mixtures contain multiple isobaric oligosaccharides, in which case this approach is often limited. Here we show how ion intensity information can be used in order to enhance MS/MS data analysis, and extract both qualitative and semiquantitative information from complex glycomic MS/MS datasets. METHODS: A matrix-assisted laser desorption/ionization quadrupole time-of-flight (MALDI QTOF) mass spectrometer was used in this study. We compared the intensities of product ions within a single product ion series, determined by their length, across the whole glycomic MS/MS dataset. In order to detect discernable patterns, the intensity data was normalized to the intensity of each product ion within the series. In most cases, normalized data yielded easily discernable patterns, relevant either for analysis of specific glycomic structure types, or mechanistic MS studies. RESULTS: We used our approach on a glycomic sample consisting of human milk oligosaccharides. The approach yielded useful results for both qualitative and semiquantitative analyses. All normalizations performed were not equally rich in information and the information content of generated tables was not possible to predict. These analyses were shown to be independent of instrument manufacturer. CONCLUSIONS: Our approach enabled more detailed qualitative analysis of MS/MS spectra of precursor ions containing isobaric oligosaccharide structures. While limited semiquantitative information could be extracted from the raw data as well, the accuracy of this method should be significantly enhanced when standard calibration mixtures can be prepared. Copyright (c) 2017 John Wiley & Sons, Ltd.
Gas-Phase Fragmentation Behavior of Oxidized Prenyl Peptides by CID and ETD Tandem Mass Spectrometry.[Pubmed:27785692]
J Am Soc Mass Spectrom. 2017 Apr;28(4):704-707.
Farnesylation and geranylgeranylation are the two types of prenyl modification of proteins. Prenylated peptides are highly hydrophobic and their abundances in biological samples are low. In this report, we studied the oxidized prenylated peptides by electrospray ionization mass spectrometry and identified them by collision-induced dissociation (CID) and electron-transfer dissociation (ETD) tandem mass spectrometry. Modified prenyl peptides were generated utilizing strong and low strength oxidizing agents to selectively oxidize and epoxidize cysteine sulfur and prenyl side chain. We selected three peptides with prenyl motifs and synthesized their prenylated versions. The detailed characteristic fragmentations of oxidized and epoxidized farnesylated and geranylgeranylated peptides were studied side by side with two popular fragmentation techniques. CID and ETD mass spectrometry clearly distinguished the modified version of these peptides. ETD mass spectrometry provided sequence information of the highly labile modified prenyl peptides and showed different characteristic fragmentations compared with CID. A detailed fragmentation of modified geranylgeranylated peptides was compared by CID and ETD mass spectrometry for the first time. Graphical Abstract .
Modeling collision energy transfer in APCI/CID mass spectra of PAHs using thermal-like post-collision internal energy distributions.[Pubmed:27802636]
J Chem Phys. 2016 Oct 28;145(16):164311.
The internal energy transferred when projectile molecular ions of naphthalene collide with argon gas atoms was extracted from the APCI-CID (atmospheric-pressure chemical ionization collision-induced dissociation) mass spectra acquired as a function of collision energy. Ion abundances were calculated by microcanonical integration of the differential rate equations using the Rice-Ramsperger-Kassel-Marcus rate constants derived from a UB3LYP/6-311G+(3df,2p)//UB3LYP/6-31G(d) fragmentation mechanism and thermal-like vibrational energy distributions pME,Tchar. The mean vibrational energy excess of the ions was characterized by the parameter Tchar ("characteristic temperature"), determined by fitting the theoretical ion abundances to the experimental breakdown graph (a plot of relative abundances of the ions as a function of kinetic energy) of activated naphthalene ions. According to these results, the APCI ion source produces species below Tchar = 1457 K, corresponding to 3.26 eV above the vibrational ground state. Subsequent collisions heat the ions up further, giving rise to a sigmoid curve of Tchar as a function of Ecom (center-of-mass-frame kinetic energy). The differential internal energy absorption per kinetic energy unit (dEvib/dEcom) changes with Ecom according to a symmetric bell-shaped function with a maximum at 6.38 +/- 0.32 eV (corresponding to 6.51 +/- 0.27 eV of vibrational energy excess), and a half-height full width of 6.30 +/- 1.15 eV. This function imposes restrictions on the amount of energy that can be transferred by collisions, such that a maximum is reached as kinetic energy is increased. This behavior suggests that the collisional energy transfer exhibits a pronounced increase around some specific value of energy. Finally, the model is tested against the CID mass spectra of anthracene and pyrene ions and the corresponding results are discussed.
Identification of small-molecule inhibitors of the colorectal cancer oncogene Kruppel-like factor 5 expression by ultrahigh-throughput screening.[Pubmed:21885866]
Mol Cancer Ther. 2011 Nov;10(11):2043-51.
The transcription factor Kruppel-like factor 5 (KLF5) is primarily expressed in the proliferative zone of the mammalian intestinal epithelium, where it regulates cell proliferation. Studies showed that inhibition of KLF5 expression reduces proliferation rates in human colorectal cancer cells and intestinal tumor formation in mice. To identify chemical probes that decrease levels of KLF5, we used cell-based ultrahigh-throughput screening (uHTS) to test compounds in the public domain of NIH, the Molecular Libraries Probe Production Centers Network library. The primary screen involved luciferase assays in the DLD-1/pGL4.18hKLF5p cell line, which stably expressed a luciferase reporter driven by the human KLF5 promoter. A cytotoxicity counterscreen was done in the rat intestinal epithelial cell line, IEC-6. We identified 97 KLF5-selective compounds with EC(50) < 10 mumol/L for KLF5 inhibition and EC(50) > 10 mumol/L for IEC-6 cytotoxicity. The two most potent compounds, CIDs (PubChem Compound IDs) 439501 and 5951923, were further characterized on the basis of computational, Western blot, and cell viability analyses. Both of these compounds, and two newly synthesized structural analogs of CID 5951923, significantly reduced endogenous KLF5 protein levels and decreased viability of several colorectal cancer cell lines without any apparent impact on IEC-6 cells. Finally, when tested in the NCI-60 panel of human cancer cell lines, compound CID 5951923 was selectively active against colon cancer cells. Our results show the feasibility of uHTS in identifying novel compounds that inhibit colorectal cancer cell proliferation by targeting KLF5.


