PiracetamModulates neurotransmission at muscarinic receptors CAS# 7491-74-9 |
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7491-74-9 | SDF | Download SDF |
| PubChem ID | 4843 | Appearance | Powder |
| Formula | C6H10N2O2 | M.Wt | 142.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (703.43 mM) H2O : ≥ 50 mg/mL (351.72 mM) *"≥" means soluble, but saturation unknown. | ||
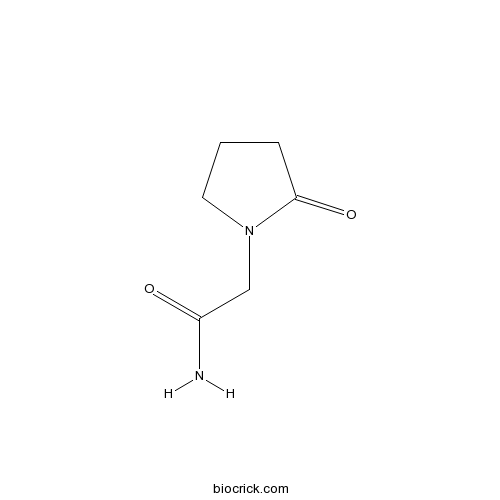
| Chemical Name | 2-(2-oxopyrrolidin-1-yl)acetamide | ||
| SMILES | C1CC(=O)N(C1)CC(=O)N | ||
| Standard InChIKey | GMZVRMREEHBGGF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H10N2O2/c7-5(9)4-8-3-1-2-6(8)10/h1-4H2,(H2,7,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nootropic that displays cognitive enhancing properties. Proposed to enhance neurotransmission via modulation of ion flux; potentiates Na+ influx through AMPA receptors. Facilitates efficiency of cholinergic neurotransmission at muscarinic receptors. |

Piracetam Dilution Calculator

Piracetam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.0343 mL | 35.1716 mL | 70.3433 mL | 140.6866 mL | 175.8582 mL |
| 5 mM | 1.4069 mL | 7.0343 mL | 14.0687 mL | 28.1373 mL | 35.1716 mL |
| 10 mM | 0.7034 mL | 3.5172 mL | 7.0343 mL | 14.0687 mL | 17.5858 mL |
| 50 mM | 0.1407 mL | 0.7034 mL | 1.4069 mL | 2.8137 mL | 3.5172 mL |
| 100 mM | 0.0703 mL | 0.3517 mL | 0.7034 mL | 1.4069 mL | 1.7586 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Piracetam is a cyclic derivative of the neurotransmitter gamma-aminobutyric acid (GABA), used in treatment of a wide range of cognitive disorders.
- 5-Acetoxymatairesinol dimethyl ether
Catalog No.:BCN4300
CAS No.:74892-45-8
- 5-Carboxamidotryptamine maleate
Catalog No.:BCC6652
CAS No.:74885-72-6
- Vernakalant Hydrochloride
Catalog No.:BCC2037
CAS No.:748810-28-8
- alpha-Carotene
Catalog No.:BCN3880
CAS No.:7488-99-5
- Argatroban
Catalog No.:BCC3723
CAS No.:74863-84-6
- Boc-Hyp-Ome
Catalog No.:BCC3252
CAS No.:74844-91-0
- GRP (porcine)
Catalog No.:BCC5809
CAS No.:74815-57-9
- 3',5'-Anhydrothymidine
Catalog No.:BCC8596
CAS No.:7481-90-5
- Zalcitabine
Catalog No.:BCC5026
CAS No.:7481-89-2
- Methylophiopogonanone A
Catalog No.:BCN5417
CAS No.:74805-92-8
- Methylophiopogonanone B
Catalog No.:BCN5418
CAS No.:74805-91-7
- Methylophiopogonone A
Catalog No.:BCN2841
CAS No.:74805-90-6
- DMAT
Catalog No.:BCC1533
CAS No.:749234-11-5
- Allyl phenoxyacetate
Catalog No.:BCC8813
CAS No.:7493-74-5
- NSC 405020
Catalog No.:BCC2120
CAS No.:7497-07-6
- 2-((1,1-Dioxidotetrahydrothiophen-3-yl)(methyl)amino)-2-oxoethyl 3-(3-nitrophenyl)acrylate
Catalog No.:BCC6185
CAS No.:749872-43-3
- JSH-23
Catalog No.:BCC4610
CAS No.:749886-87-1
- Callimorphine
Catalog No.:BCN1959
CAS No.:74991-73-4
- 9-Methoxycanthin-6-one
Catalog No.:BCN2993
CAS No.:74991-91-6
- Ethylamine
Catalog No.:BCN1799
CAS No.:75-04-7
- 1,2-Cyclohexanedicarboximide
Catalog No.:BCC8416
CAS No.:7506-66-3
- Daphnilongeranin C
Catalog No.:BCN4301
CAS No.:750649-07-1
- 20,24-Dihydroxydammar-25-en-3-one
Catalog No.:BCN4302
CAS No.:75069-59-9
- 2',6'-Dihydroxy-4'-methoxyacetophenone
Catalog No.:BCN6891
CAS No.:7507-89-3
Piracetam Facilitates the Anti-Amnesic but not Anti-Diabetic Activity of Metformin in Experimentally Induced Type-2 Diabetic Encephalopathic Rats.[Pubmed:27585927]
Cell Mol Neurobiol. 2017 Jul;37(5):791-802.
Piracetam exhibits anti-amnesic activity in several animal models of dementia. However, its anti-amnesic potential has yet to be evaluated in type-2 diabetes mellitus (T2DM)-induced encephalopathy. Therefore, in the present study, Piracetam (25, 50 and 100 mg/kg) was screened for anti-amnesic and anti-diabetic activity in T2DM-induced encephalopathic male rats. Subsequently, anti-amnesic and anti-diabetic activities were evaluated for Piracetam, metformin and their combination in T2DM-induced encephalopathic animals. Rats received streptozotocin (45 mg/kg) and nicotinamide (110 mg/kg) injections on day-1 (D-1) of the experimental schedule and were kept undisturbed for 35 days to exhibit T2DM-induced encephalopathy. All drug treatments were continued from D-7 to D-35 in both experiments. Piracetam (100 mg/kg) attenuated loss in learning and memory in terms of increase in escape latency on D-4 (D-34) and decrease in time spent in the target quadrant on D-5 (D-35) of Morris water maze test protocol, and spatial memory in terms of reduced spontaneous alternation behavior in Y-maze test of encephalopathic rats. Additionally, Piracetam attenuated altered levels of fasting plasma glucose and insulin, HOMA-IR and HOMA-B in encephalopathic animals, comparatively lesser than metformin. In the next experiment, combination of Piracetam and metformin exhibited better anti-amnesic but not anti-diabetic activity than respective monotherapies in encephalopathic rats. Further, the combination attenuated reduced acetylcholine level and increased acetylcholinesterase activity, increased glycogen synthase kinase-3beta level and decreased brain-derived neurotropic factor level in hippocampus and pre-frontal cortex of encephalopathic animals. Thus, Piracetam could be used as an adjuvant to metformin in the management of dementia in T2DM-induced encephalopathy.
Stability-Indicating TLC-Densitometric and HPLC Methods for the Simultaneous Determination of Piracetam and Vincamine in the Presence of Their Degradation Products.[Pubmed:27569579]
J AOAC Int. 2016 Nov 1;99(6):1490-1498.
Newly established TLC-densitometric and RP-HPLC methods were developed and validated for the simultaneous determination of Piracetam (PIR) and Vincamine (VINC) in their pharmaceutical formulation and in the presence of PIR and VINC degradation products, PD and VD, respectively. The proposed TLC-densitometric method is based on the separation and quantitation of the studied components using a developing system that consists of chloroform-methanol-glacial acetic acid-triethylamine (8 + 2 + 0.1 + 0.1, v/v/v/v) on TLC silica gel 60 F254 plates, followed by densitometric scanning at 230 nm. On the other hand, the developed RP-HPLC method is based on the separation of the studied components using an isocratic elution of 0.05 M KH2PO4 (containing 0.1% triethylamine adjusted to pH 3 with orthophosphoric acid)-methanol (95 + 5, v/v) on a C8 column at a flow rate of 1 mL/min with diode-array detection at 230 nm. The developed methods were validated according to International Conference on Harmonization guidelines and demonstrated good accuracy and precision. Moreover, the developed TLC-densitometric and RP-HPLC methods are suitable as stability-indicating assay methods for the simultaneous determination of PD and VD either in bulk powder or pharmaceutical formulation. The results were statistically compared with those obtained by the reported RP-HPLC method using t- and F-tests.
Enhanced Neuroplasticity by the Metabolic Enhancer Piracetam Associated with Improved Mitochondrial Dynamics and Altered Permeability Transition Pore Function.[Pubmed:27747106]
Neural Plast. 2016;2016:8075903.
The mitochondrial cascade hypothesis of dementia assumes mitochondrial dysfunction leading to reduced energy supply, impaired neuroplasticity, and finally cell death as one major pathomechanism underlying the continuum from brain aging over mild cognitive impairment to initial and advanced late onset Alzheimer's disease. Accordingly, improving mitochondrial function has become an important strategy to treat the early stages of this continuum. The metabolic enhancer Piracetam has been proposed as possible prototype for those compounds by increasing impaired mitochondrial function and related aspects like mechanisms of neuroplasticity. We here report that Piracetam at therapeutically relevant concentrations improves neuritogenesis in the human cell line SH-SY5Y over conditions mirroring the whole spectrum of age-associated cognitive decline. These effects go parallel with improvement of impaired mitochondrial dynamics shifting back fission and fusion balance to the energetically more favorable fusion site. Impaired fission and fusion balance can also be induced by a reduction of the mitochondrial permeability transition pore (mPTP) function as atractyloside which indicates the mPTP has similar effects on mitochondrial dynamics. These changes are also reduced by Piracetam. These findings suggest the mPTP as an important target for the beneficial effects of Piracetam on mitochondrial function.
Piracetam Attenuates LPS-Induced Neuroinflammation and Cognitive Impairment in Rats.[Pubmed:28176051]
Cell Mol Neurobiol. 2017 Nov;37(8):1373-1386.
The present study was performed to investigate the effect of Piracetam on neuroinflammation induced by lipopolysaccharide (LPS) and resulting changes in cognitive behavior. Neuroinflammation was induced by a single dose of LPS solution infused into each of the lateral cerebral ventricles in concentrations of 1 mug/mul, at a rate of 1 mul/min over a 5-min period, with a 5-min waiting period between the two infusions. Piracetam in doses of 50, 100, and 200 mg/kg i.p. was administered 30 min before LPS infusion and continued for 9 days. On ninth day, the behavioral test for memory and anxiety was done followed by blood collection and microdissection of the hippocampus (HIP) and prefrontal cortex brain regions. Piracetam attenuated the LPS-induced decrease in coping strategy to novel environment indicating anxiolytic activity. It also reversed the LPS-induced changes in the known arm and novel arm entries in the Y-maze test indicating amelioration of spatial memory impairment. Further, Piracetam moderated LPS-induced decrease in the mitochondrial complex enzyme activities (I, II, IV, and V) and mitochondrial membrane potential. It ameliorated changes in hippocampal lipid peroxidation and nitrite levels including the activity of superoxide dismutase. Piracetam region specifically ameliorated LPS-induced increase in the level of IL-6 in HIP indicating anti-neuroinflammatory effect. Further, Piracetam reduced HIP Abeta (1-40) and increased blood Abeta level suggesting efflux of Abeta from HIP to blood. Therefore, the present study indicates preclinical evidence for the use of Piracetam in the treatment of neuroinflammatory disorders.
Piracetam: a review of pharmacological properties and clinical uses.[Pubmed:16007238]
CNS Drug Rev. 2005 Summer;11(2):169-82.
Piracetam, a derivative of the neurotransmitter gamma-aminobutyric acid (GABA), has a variety of physiological effects that may result, at least in part, from the restoration of cell membrane fluidity. At a neuronal level, Piracetam modulates neurotransmission in a range of transmitter systems (including cholinergic and glutamatergic), has neuroprotective and anticonvulsant properties, and improves neuroplasticity. At a vascular level, it appears to reduce erythrocyte adhesion to vascular endothelium, hinder vasospasm, and facilitate microcirculation. This diverse range of physiological effects is consistent with its use in a range of clinical indications. Its efficacy is documented in cognitive disorders and dementia, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia. While high doses are sometimes necessary, Piracetam is well tolerated.
Piracetam and other structurally related nootropics.[Pubmed:8061686]
Brain Res Brain Res Rev. 1994 May;19(2):180-222.
Nearly three decades have now passed since the discovery of the Piracetam-like nootropics, compounds which exhibit cognition-enhancing properties, but for which no commonly accepted mechanism of action has been established. This review covers clinical, pharmacokinetic, biochemical and behavioural results presented in the literature from 1965 through 1992 (407 references) of Piracetam, oxiracetam, pramiracetam, etiracetam, nefiracetam, aniracetam and rolziracetam and their structural analogues. The Piracetam-like nootropics are capable of achieving reversal of amnesia induced by, e.g., scopolamine, electroconvulsive shock and hypoxia. Protection against barbiturate intoxication is observed and some benefit in clinical studies with patients suffering from mild to moderate degrees of dementia has been demonstrated. No affinity for the alpha 1-, alpha 2-, beta-, muscarinic, 5-hydroxytryptamine-, dopamine, adenosine-A1-, mu-opiate, gamma-aminobutyric acid (GABA) (except for nefiracetam (GABAA)), benzodiazepine and glutamate receptors has been found. The racetams possess a very low toxicity and lack serious side effects. Increased turnover of different neurotransmitters has been observed as well as other biochemical findings, e.g., inhibition of enzymes such as prolylendopeptidase. So far, no generally accepted mechanism of action has, however, emerged. We believe that the effect of the racetams is due to a potentiation of already present neurotransmission and that much evidence points in the direction of a modulated ion flux by, e.g., potentiated calcium influx through non-L-type voltage-dependent calcium channels, potentiated sodium influx through alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor gated channels or voltage-dependent channels or decreases in potassium efflux. Effects on carrier mediated ion transport are also possible.
Piracetam. An overview of its pharmacological properties and a review of its therapeutic use in senile cognitive disorders.[Pubmed:1794001]
Drugs Aging. 1991 Jan;1(1):17-35.
Piracetam is the first of the so-called 'nootropic' drugs, a unique class of drugs which affect mental function. In animal models and in healthy volunteers, the drug improves the efficiency of the higher telencephalic functions of the brain involved in cognitive processes such as learning and memory. The pharmacology of Piracetam is unusual because it protects against various physical and chemical insults applied to the brain. It facilitates learning and memory in healthy animals and in animals whose brain function has been compromised, and it enhances interhemispheric transfer of information via callosal transmission. At the same time, even in relatively high dosages it is devoid of any sedative, analeptic or autonomic activities. How Piracetam exerts its effects on memory disorders is still under investigation, although among other proposed mechanisms of action it is thought to facilitate central nervous system efficiency of cholinergic neurotransmission. Results from trials involving elderly patients with senile cognitive disorders have been equivocal, as have the results obtained when Piracetam has been combined with acetylcholine precursors. Piracetam seems to be almost completely devoid of adverse effects, and is extremely well tolerated. In conclusion, opinion is divided as to the benefits of Piracetam in the treatment of senile cognitive decline. Although double-blind studies in the elderly have produced mixed results, some such trials (particularly those involving larger numbers of patients) have reported favourable findings, thus offering some reason for cautious optimism in a notoriously difficult area of therapeutics. However, further investigations of Piracetam alone and in combination therapy are required before any absolute conclusions can be drawn.


