ArgatrobanThrombin mediated fibrinogen cleavage inhibitor CAS# 74863-84-6 |
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- PD98059
Catalog No.:BCC1098
CAS No.:167869-21-8
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 74863-84-6 | SDF | Download SDF |
| PubChem ID | 440542 | Appearance | Powder |
| Formula | C23H36N6O5S | M.Wt | 508.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Argipidine | ||
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in 1eq. HCl | ||
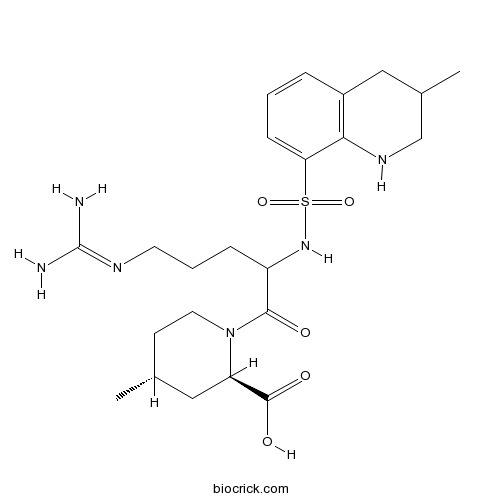
| Chemical Name | (2R,4R)-1-[5-(diaminomethylideneamino)-2-[(3-methyl-1,2,3,4-tetrahydroquinolin-8-yl)sulfonylamino]pentanoyl]-4-methylpiperidine-2-carboxylic acid | ||
| SMILES | CC1CCN(C(C1)C(=O)O)C(=O)C(CCCN=C(N)N)NS(=O)(=O)C2=CC=CC3=C2NCC(C3)C | ||
| Standard InChIKey | KXNPVXPOPUZYGB-VSVYTNTFSA-N | ||
| Standard InChI | InChI=1S/C23H36N6O5S/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26)/t14-,15?,17?,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of thrombin mediated fibrinogen cleavage (Ki = 19 nM). Competitive inhibitor of thrombin-induced platelet activation and clotting. Shown to exhibit antithrombotic activity in animal models. |

Argatroban Dilution Calculator

Argatroban Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9661 mL | 9.8303 mL | 19.6607 mL | 39.3213 mL | 49.1516 mL |
| 5 mM | 0.3932 mL | 1.9661 mL | 3.9321 mL | 7.8643 mL | 9.8303 mL |
| 10 mM | 0.1966 mL | 0.983 mL | 1.9661 mL | 3.9321 mL | 4.9152 mL |
| 50 mM | 0.0393 mL | 0.1966 mL | 0.3932 mL | 0.7864 mL | 0.983 mL |
| 100 mM | 0.0197 mL | 0.0983 mL | 0.1966 mL | 0.3932 mL | 0.4915 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Potent inhibitor of thrombin mediated fibrinogen cleavage (Ki = 19 nM). Competitive inhibitor of thrombin-induced platelet activation and clotting. Shown to exhibit antithrombotic activity in animal models.
- Boc-Hyp-Ome
Catalog No.:BCC3252
CAS No.:74844-91-0
- GRP (porcine)
Catalog No.:BCC5809
CAS No.:74815-57-9
- 3',5'-Anhydrothymidine
Catalog No.:BCC8596
CAS No.:7481-90-5
- Zalcitabine
Catalog No.:BCC5026
CAS No.:7481-89-2
- Methylophiopogonanone A
Catalog No.:BCN5417
CAS No.:74805-92-8
- Methylophiopogonanone B
Catalog No.:BCN5418
CAS No.:74805-91-7
- Methylophiopogonone A
Catalog No.:BCN2841
CAS No.:74805-90-6
- Methylophiopogonone B
Catalog No.:BCN8182
CAS No.:74805-89-3
- Ciglitazone
Catalog No.:BCC7014
CAS No.:74772-77-3
- VER-50589
Catalog No.:BCC5296
CAS No.:747413-08-7
- AUY922 (NVP-AUY922)
Catalog No.:BCC2123
CAS No.:747412-49-3
- Pterolactone A
Catalog No.:BCN6513
CAS No.:74730-10-2
- alpha-Carotene
Catalog No.:BCN3880
CAS No.:7488-99-5
- Vernakalant Hydrochloride
Catalog No.:BCC2037
CAS No.:748810-28-8
- 5-Carboxamidotryptamine maleate
Catalog No.:BCC6652
CAS No.:74885-72-6
- 5-Acetoxymatairesinol dimethyl ether
Catalog No.:BCN4300
CAS No.:74892-45-8
- Piracetam
Catalog No.:BCC4824
CAS No.:7491-74-9
- DMAT
Catalog No.:BCC1533
CAS No.:749234-11-5
- Allyl phenoxyacetate
Catalog No.:BCC8813
CAS No.:7493-74-5
- NSC 405020
Catalog No.:BCC2120
CAS No.:7497-07-6
- 2-((1,1-Dioxidotetrahydrothiophen-3-yl)(methyl)amino)-2-oxoethyl 3-(3-nitrophenyl)acrylate
Catalog No.:BCC6185
CAS No.:749872-43-3
- JSH-23
Catalog No.:BCC4610
CAS No.:749886-87-1
- Callimorphine
Catalog No.:BCN1959
CAS No.:74991-73-4
- 9-Methoxycanthin-6-one
Catalog No.:BCN2993
CAS No.:74991-91-6
ISMP Medication Error Report Analysis: Aggrastat-Argatroban Mix-ups Don't Expect Radiofrequency Identification Stock Systems To Be Perfect Paralyzed by Mistakes: Reassess the Safety of Neuromuscular Blockers in Your Facility.[Pubmed:28057945]
Hosp Pharm. 2016 Dec;51(11):877-883.
These medication errors have occurred in health care facilities at least once. They will happen again-perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs. Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers' names will be published if desired. ISMP may be contacted at the address shown below. Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters' wishes as to the level of detail included in publications.
Monitoring of Argatroban and Lepirudin: What is the Input of Laboratory Values in "Real Life"?[Pubmed:28320219]
Clin Appl Thromb Hemost. 2018 Mar;24(2):287-294.
Monitoring of direct thrombin inhibitors (DTIs) in patients with heparin-induced thrombocytopenia (HIT) is primarily performed using the activated partial thromboplastin time (aPTT). This assay is poorly standardized, reagent dependent, and not DTI specific. We compared aPTT, thrombin time (TT), and prothrombin time (PT) to drug levels obtained by the ecarin chromogenic assay (ECA). We analyzed 495 samples of patients with confirmed or suspected HIT on treatment with either Argatroban (n = 37) or lepirudin (n = 80). Mean DTI levels +/- standard deviation (SD) were 0.41 +/- 0.36 microg/mL for Argatroban and 0.20 +/- 0.21 microg/mL for lepirudin. Results of aPTT were highly variable: 67 +/- 22 seconds for Argatroban and 55 +/- 20 seconds for lepirudin. Significant correlations ( P < .01) were found between ECA-based DTI level and TT (Argatroban, r = .820 and lepirudin, r = .830), PT (Argatroban, r = -.544), and aPTT (lepirudin, r = .572). However, there was no correlation of aPTT with Argatroban or PT with lepirudin concentration. Multiple regression analyses revealed that the TT predicted 54% of Argatroban and 42% of lepirudin levels, but no significant impact was seen for PT or aPTT. The aPTT-guided monitoring of DTI therapy leads to a high percentage of patients with inaccurate plasma levels, hence resulting to either undertreatment or overtreatment. Knowledge of baseline values prior to DTI therapy and inclusion of clinical settings are essential for dosing DTIs when using aPTT. However, due to several limitations of aPTT, monitoring according to exact plasma concentrations as obtained by specific tests such as ECA may be more appropriate.
Biodegradable polymer-based, argatroban-eluting, cobalt-chromium stent (JF-04) for treatment of native coronary lesions: final results of the first-in-man study and lessons learned.[Pubmed:27866136]
EuroIntervention. 2016 Nov 20;12(10):1271-1278.
AIMS: The aim of the study was to investigate the six-month angiographic and nine-month clinical follow-up outcomes in a first-in-man study using the biodegradable polymer-based cobalt-chromium Argatroban-eluting stent (JF-04) for treatment of native coronary atherosclerotic lesions. METHODS AND RESULTS: A total of 31 patients with either stable or unstable angina, or silent myocardial ischaemia, exhibiting de novo coronary lesions were enrolled at seven Japanese sites. The lesions were treated with the JF-04 stent after predilatation. The primary endpoint was angiographic in-stent late loss six months after implantation. The secondary endpoints included angiographic restenosis and in-stent volume obstruction by intravascular ultrasound at six months and target vessel failure (TVF) at nine months. Procedural success was achieved in 100% of cases. At six months, angiographic in-stent late loss was 1.01+/-0.48 mm and binary restenosis was observed in nine cases (29.0%). Among these restenotic cases, most (n=8) demonstrated advanced angiographic restenosis patterns, including diffuse/proliferative restenosis and total occlusion. At nine months, TVF was observed in four cases (12.9%), exclusively attributed to target vessel revascularisation. CONCLUSIONS: This Argatroban-eluting stent failed to inhibit neointimal hyperplasia sufficiently, despite the theoretical benefits and promising clinical experience with local drug delivery.
Prevention of platelet-rich arterial thrombosis by selective thrombin inhibition.[Pubmed:2297828]
Circulation. 1990 Jan;81(1):219-25.
The effect of heparin and of the synthetic competitive thrombin inhibitor (2R,4R)-4-methyl-1-[N2-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfon yl)-L-arginyl]-2-piperidinecarboxylic acid monohydrate (Argatroban) on platelet-rich arterial thrombosis was studied in a rabbit model, consisting of a 4-6-mm everted ("inside-out") femoral arterial segment. Intravenous injection of heparin (200 units/kg) failed to prevent occlusion within 60 minutes in all 10 rabbits, whereas intravenous Argatroban infusion at a rate of 100 or 200 micrograms/kg/min for 60 minutes, which prolonged the thrombin time more than fourfold, prevented thrombosis in nine of 13 rabbits (p = 0.002 vs. i.v. heparin). Intra-arterial infusion of 200 units/kg heparin over 60 minutes prevented occlusion in six of nine rabbits (p = 0.003 vs. i.v. heparin), whereas intra-arterial Argatroban at a rate of 100 micrograms/kg/min for 60 minutes prevented thrombosis in all 10 rabbits (p = 0.00001 vs. i.v. heparin). Patency of femoral arterial segments was maintained after the end of the intra-arterial heparin and intravenous or intra-arterial Argatroban infusion for up to 3 hours despite normalization of the thrombin time and partial thromboplastin time. Pathologic examination of the graft revealed that the inverted adventitial surface was covered by layers of platelets without platelet aggregation or fibrin deposition. These findings indicate that thrombin plays an important role in platelet-rich arterial thrombosis, and that the thrombogenic stimulus is rapidly attenuated by short-term infusion of the synthetic thrombin inhibitor. Selective thrombin inhibition can constitute an alternative approach to the prevention of arterial occlusion after angioplasty or thrombolytic therapy in patients with unstable coronary syndromes.
Selective inhibition of thrombin by (2R,4R)-4-methyl-1-[N2-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl++ +) sulfonyl]-l-arginyl)]-2-piperidinecarboxylic acid.[Pubmed:6691968]
Biochemistry. 1984 Jan 3;23(1):85-90.
The potency of thrombin inhibition by 4-methyl-1-[N2-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)-sulfony l]- L-arginyl]-2-piperidinecarboxylic acid (MQPA) depended on the stereoconformation of the 2-piperidinecarboxylic acid moiety. Ki values for bovine alpha-thrombin were 0.019 microM with (2R,4R)-MQPA, 0.24 microM with (2R,4S)-MQPA, 1.9 microM with (2S,4R)-MQPA, and 280 microM with (2S,4S)-MQPA. (2R,4R)-MQPA of the four stereoisomers of MQPA was also the most potent inhibitor for other trypsin-like serine proteases with Ki values of 5.0 microM for trypsin, 210 microM for factor Xa, 800 microM for plasmin, and 1500 microM for plasma kallikrein. Examination of the potency of thrombin inhibition by arginine derivatives related to MQPA in structure suggested the presence of a specific binding site for the carboxamide portion (C-terminal side). The relative inhibitory potency of the four stereoisomers of MQPA for trypsin was nearly identical with that for thrombin, suggesting that the specific binding site for the carboxamide portion is present in both enzymes. Modification of thrombin by phosphopyridoxylation or the presence of heparin did not significantly alter the binding of MQPA.


