BMS 453RAR agonist CAS# 166977-43-1 |
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
- CD 2314
Catalog No.:BCC6071
CAS No.:170355-37-0
- CD 2665
Catalog No.:BCC7778
CAS No.:170355-78-9
- AGN 194310
Catalog No.:BCC5416
CAS No.:229961-45-9
- TTNPB (Arotinoid Acid)
Catalog No.:BCC4874
CAS No.:71441-28-6
- AC 261066
Catalog No.:BCC7848
CAS No.:870773-76-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 166977-43-1 | SDF | Download SDF |
| PubChem ID | 9875424 | Appearance | Powder |
| Formula | C27H24O2 | M.Wt | 380.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMS 189453 | ||
| Solubility | Soluble to 100 mM in DMSO | ||
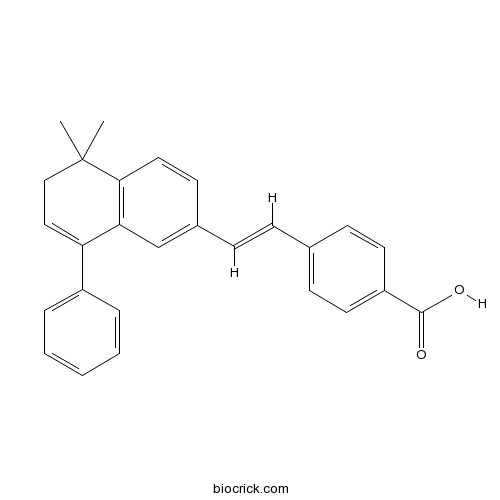
| Chemical Name | 4-[(E)-2-(5,5-dimethyl-8-phenyl-6H-naphthalen-2-yl)ethenyl]benzoic acid | ||
| SMILES | CC1(CC=C(C2=C1C=CC(=C2)C=CC3=CC=C(C=C3)C(=O)O)C4=CC=CC=C4)C | ||
| Standard InChIKey | VUODRPPTYLBGFM-CMDGGOBGSA-N | ||
| Standard InChI | InChI=1S/C27H24O2/c1-27(2)17-16-23(21-6-4-3-5-7-21)24-18-20(12-15-25(24)27)9-8-19-10-13-22(14-11-19)26(28)29/h3-16,18H,17H2,1-2H3,(H,28,29)/b9-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Synthetic retinoid. Retinoic acid receptor β (RARβ) agonist in vivo; RARα and RARγ antagonist in vitro. Inhibits breast cell proliferation by inducing active transforming growth factor β (TGFβ) and causes G1 arrest. |

BMS 453 Dilution Calculator

BMS 453 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6283 mL | 13.1413 mL | 26.2826 mL | 52.5652 mL | 65.7065 mL |
| 5 mM | 0.5257 mL | 2.6283 mL | 5.2565 mL | 10.513 mL | 13.1413 mL |
| 10 mM | 0.2628 mL | 1.3141 mL | 2.6283 mL | 5.2565 mL | 6.5706 mL |
| 50 mM | 0.0526 mL | 0.2628 mL | 0.5257 mL | 1.0513 mL | 1.3141 mL |
| 100 mM | 0.0263 mL | 0.1314 mL | 0.2628 mL | 0.5257 mL | 0.6571 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMS 453 is a synthetic RAR (retinoic acid receptor) antagonist [1].
The retinoic acid receptors (RARα, RARβ, RARγ) belong to steroid nuclear receptor superfamily and the expressions of retinoid receptor RARα and RARβ are correlated with the antiproliferative effects of retinoids.
In normal breast cells, BMS453 inhibited the cell proliferation without significantly apoptosis. BMS 453 blocked the G1/S transition of the cell cycle with an increase in the amount of cells in G0/G1 and a decrease in the amount of cells in S phase. Also, BMS453 increased active TGFβ activity by 33-fold, which show BMS453 induces conversion of latent TGFβ to active TGFβ. TGFβ mediates the anti-proliferative effect of BMS453 in breast cells [1]. BMS 453 transrepresses AP-1 but does not activate RAR-dependent gene expression. In normal breast cells (HMEC and 184) and T47D breast cancer cells, BMS453 inhibited the growth of T47D breast cancer cells and normal breast cells (HMEC and 184). Breast cancer cells were resistant to BMS453 and normal human breast cells were most sensitive to BMS453. BMS453 may suppress growth by inhibiting transcription factors such as AP-1 [2].
References:
[1]. Yang L, Ostrowski J, Reczek P, et al. The retinoic acid receptor antagonist, BMS453, inhibits normal breast cell growth by inducing active TGFbeta and causing cell cycle arrest. Oncogene, 2001, 20(55): 8025-8035.
[2]. Yang L, Munoz-Medellin D, Kim HT, et al. Retinoic acid receptor antagonist BMS453 inhibits the growth of normal and malignant breast cells without activating RAR-dependent gene expression. Breast Cancer Res Treat, 1999, 56(3): 277-291.
- N-Acetoacetylmorpholine
Catalog No.:BCC9078
CAS No.:16695-54-8
- Tetramethylkaempferol
Catalog No.:BCN8082
CAS No.:16692-52-7
- 3-Amino-5-mercapto-1,2,4-triazole
Catalog No.:BCC8615
CAS No.:16691-43-3
- A-1210477
Catalog No.:BCC6508
CAS No.:1668553-26-1
- Chebulanin
Catalog No.:BCN3261
CAS No.:166833-80-3
- H-D-Orn-OH. HCl
Catalog No.:BCC3004
CAS No.:16682-12-5
- H-Gly-NH2.HCl
Catalog No.:BCC2947
CAS No.:1668-10-6
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
- Prerubialatin
Catalog No.:BCN6895
CAS No.:1667718-89-9
- Z-Tyr(Bzl)-OH
Catalog No.:BCC2735
CAS No.:16677-29-5
- Naltrexone HCl
Catalog No.:BCC4613
CAS No.:16676-29-2
- H-Cys(Trt)-NH2
Catalog No.:BCC2912
CAS No.:166737-85-5
- Fmoc-D-Cys(Trt)-OH
Catalog No.:BCC3481
CAS No.:167015-11-4
- Pitavastatin ethyl ester
Catalog No.:BCC9122
CAS No.:167073-19-0
- 10-Oxo Docetaxel
Catalog No.:BCC5409
CAS No.:167074-97-7
- Autocamtide-2-related inhibitory peptide
Catalog No.:BCC7153
CAS No.:167114-91-2
- R-96544 hydrochloride
Catalog No.:BCC7164
CAS No.:167144-80-1
- Furagin
Catalog No.:BCC1582
CAS No.:1672-88-4
- Clevidipine Butyrate
Catalog No.:BCC4401
CAS No.:167221-71-8
- Cyanidin 3-Sophoroside-5-Glucoside
Catalog No.:BCC8158
CAS No.:16727-02-9
- Malvidin 3,5-Diglucoside
Catalog No.:BCC8206
CAS No.:16727-30-3
- 9-Hydroxyeriobofuran
Catalog No.:BCN7444
CAS No.:167278-41-3
- Zosuquidar
Catalog No.:BCC2074
CAS No.:167354-41-8
- Fmoc-Lys(Mtt)-OH
Catalog No.:BCC3523
CAS No.:167393-62-6
Commitment of mouse embryonic stem cells to the adipocyte lineage requires retinoic acid receptor beta and active GSK3.[Pubmed:18690793]
Stem Cells Dev. 2009 Apr;18(3):457-63.
Key events leading to terminal differentiation of preadipocytes into adipocytes have been identified in recent years. However, signaling pathways involved in the decision of stem cells to follow the adipogenic lineage have not yet been characterized. We have previously shown that differentiating mouse embryonic stem (mES) cells give rise to functional adipocytes upon an early treatment with retinoic acid (RA). The goal of this work was to identify regulators of RA-induced commitment of mES cells to the adipocyte lineage. First, we investigated the role of RA receptor (RAR) isotypes in the induction of mES cell adipogenesis. Using synthetic retinoids selective of RAR isotypes, we show that RARbeta activation is both sufficient and necessary to trigger commitment of mES cells to adipocytes. Then, we performed a small-scale drug screening to find signaling pathways involved in RARbeta-induced mES cell adipogenesis. We show that pharmacological inhibitors of glycogen synthase kinase (GSK) 3, completely inhibit RARbeta-induced adipogenesis in mES cells. This finding uncovers the requirement of active GSK3 in RARbeta-induced commitment of mES cells toward the adipocyte lineage. Finally, we investigated the role of the Wnt pathway, in which GSK3 is a critical negative regulator, in adipocyte commitment by analyzing Wnt pathway activity in RA- and RARbeta-induced mES cell adipogenesis. Our results suggest that although RARbeta and active GSK3 are required for RA-induced adipogenesis, they might be acting through a Wnt pathway-independent mechanism.
The retinoic acid receptor antagonist, BMS453, inhibits normal breast cell growth by inducing active TGFbeta and causing cell cycle arrest.[Pubmed:11753686]
Oncogene. 2001 Nov 29;20(55):8025-35.
We have previously shown that a retinoic acid receptor (RAR) antagonist BMS453, which does not activate RAR-dependent gene transcription in breast cells, inhibits normal breast cell growth. In this study we have investigated the mechanisms by which this retinoid receptor antagonist inhibits cell growth. Both all trans retinoic acid (atRA) and BMS453 inhibited the proliferation of normal breast cell growth without significantly inducing apoptosis. Both retinoids caused a G1 block in the cell cycle with an increase in the proportion of cells in G0/G1 and a decrease in the proportion of cells in S phase. We then investigated the effects of the retinoids on molecules that regulate the G1 to S transition. These studies demonstrated that both atRA and BMS453 induce Rb hypophosphorylation and decrease CDK2 kinase activity. We then studied the effect of the retinoids on the expression of CDK inhibitors. atRA and BMS453 increased total p21 protein levels and CDK2-bound p21 protein, but did not change CDK4-bound p21. These results suggest that atRA and BMS453 increase p21, decrease CDK2 kinase activity, which in turn leads to hypophosphorylation of Rb and G1 arrest. Because transforming growth factor beta (TGFbeta) has been proposed as a mediator of retinoid-induced growth inhibition, we next investigated whether TGFbeta mediates the anti-proliferative effect of atRA and BMS453 in normal breast cells. These studies showed that atRA and BMS453 increased total TGFbeta activity by 3-5-fold. However, BMS453 increased active TGFbeta activity by 33-fold while atRA increased active TGFbeta activity by only threefold. These results suggest that BMS453 treatment induces conversion of latent TGFbeta to active TGFbeta. To investigate whether this increase in active TGFbeta mediates the anti-proliferative effects of these retinoids, a TGFbeta-blocking antibody was used in an attempt to prevent retinoid-induced growth inhibition. Results from these experiments showed that the anti-TGFbeta antibody prevented the inhibition of cell proliferation induced by BMS453, but did not prevent the inhibition of cell proliferation induced by atRA. These results demonstrate that BMS453 inhibits breast cell growth predominantly through the induction of active TGFbeta, while atRA inhibits growth through other mechanisms. These results suggest that retinoid analogs that increase active TGFbeta may be promising agents for the prevention of breast cancer.
RAR-specific agonist/antagonists which dissociate transactivation and AP1 transrepression inhibit anchorage-independent cell proliferation.[Pubmed:7720709]
EMBO J. 1995 Mar 15;14(6):1187-97.
Using retinoic acid receptor (RAR) reporter cells specific for either RAR alpha, beta or gamma, we have identified synthetic retinoids which specifically induce transactivation by RAR beta, while antagonizing RA-induced transactivation by RAR alpha and RAR gamma. Like RA, these synthetic retinoids allow all three RAR types to repress AP1 (c-Jun/c-Fos) activity, demonstrating that the transactivation and transrepression functions of RARs can be dissociated by properly designed ligands. Using AP1 reporter cells, we also show that glucocorticoids or vitamin D3, together with either RA or these 'dissociating' synthetic retinoids, can synergistically repress phorbol ester-induced AP1 activity. RA, but not these 'dissociating' retinoids, induced transcription of an interleukin-6 promoter-based reporter gene transiently transfected into HeLa cells together with RARs. Using Ki-ras-transformed 3T3 cells as a model system, we show that both RA and the 'dissociating' retinoids inhibit anchorage-independent cell proliferation, suggesting that retinoid-induced growth inhibition may be related to AP1 transrepression.


