Clevidipine ButyrateCAS# 167221-71-8 |
- Pemirolast potassium
Catalog No.:BCC4532
CAS No.:100299-08-9
- Desloratadine
Catalog No.:BCC4540
CAS No.:100643-71-8
- Clemastine Fumarate
Catalog No.:BCC4528
CAS No.:14976-57-9
- Hydroxyzine 2HCl
Catalog No.:BCC4519
CAS No.:2192-20-3
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Brompheniramine hydrogen maleate
Catalog No.:BCC4515
CAS No.:980-71-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 167221-71-8 | SDF | Download SDF |
| PubChem ID | 153994 | Appearance | Powder |
| Formula | C21H23Cl2NO6 | M.Wt | 456.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (109.57 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5-O-(butanoyloxymethyl) 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | ||
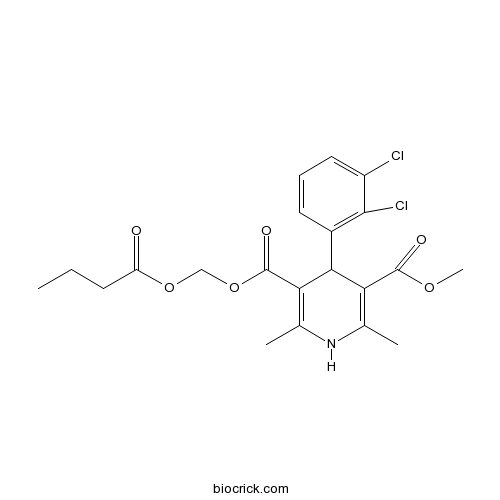
| SMILES | CCCC(=O)OCOC(=O)C1=C(NC(=C(C1C2=C(C(=CC=C2)Cl)Cl)C(=O)OC)C)C | ||
| Standard InChIKey | KPBZROQVTHLCDU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H23Cl2NO6/c1-5-7-15(25)29-10-30-21(27)17-12(3)24-11(2)16(20(26)28-4)18(17)13-8-6-9-14(22)19(13)23/h6,8-9,18,24H,5,7,10H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Clevidipine Butyrate Dilution Calculator

Clevidipine Butyrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1914 mL | 10.9572 mL | 21.9144 mL | 43.8289 mL | 54.7861 mL |
| 5 mM | 0.4383 mL | 2.1914 mL | 4.3829 mL | 8.7658 mL | 10.9572 mL |
| 10 mM | 0.2191 mL | 1.0957 mL | 2.1914 mL | 4.3829 mL | 5.4786 mL |
| 50 mM | 0.0438 mL | 0.2191 mL | 0.4383 mL | 0.8766 mL | 1.0957 mL |
| 100 mM | 0.0219 mL | 0.1096 mL | 0.2191 mL | 0.4383 mL | 0.5479 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Clevidipine is a short-acting dihydropyridine calcium channel antagonist (IC50= 7.1 nM, V(H) = -40 mV ) under development for treatment of perioperative hypertension.
- Furagin
Catalog No.:BCC1582
CAS No.:1672-88-4
- R-96544 hydrochloride
Catalog No.:BCC7164
CAS No.:167144-80-1
- Autocamtide-2-related inhibitory peptide
Catalog No.:BCC7153
CAS No.:167114-91-2
- 10-Oxo Docetaxel
Catalog No.:BCC5409
CAS No.:167074-97-7
- Pitavastatin ethyl ester
Catalog No.:BCC9122
CAS No.:167073-19-0
- Fmoc-D-Cys(Trt)-OH
Catalog No.:BCC3481
CAS No.:167015-11-4
- BMS 453
Catalog No.:BCC7679
CAS No.:166977-43-1
- N-Acetoacetylmorpholine
Catalog No.:BCC9078
CAS No.:16695-54-8
- Tetramethylkaempferol
Catalog No.:BCN8082
CAS No.:16692-52-7
- 3-Amino-5-mercapto-1,2,4-triazole
Catalog No.:BCC8615
CAS No.:16691-43-3
- A-1210477
Catalog No.:BCC6508
CAS No.:1668553-26-1
- Chebulanin
Catalog No.:BCN3261
CAS No.:166833-80-3
- Cyanidin 3-Sophoroside-5-Glucoside
Catalog No.:BCC8158
CAS No.:16727-02-9
- Malvidin 3,5-Diglucoside
Catalog No.:BCC8206
CAS No.:16727-30-3
- 9-Hydroxyeriobofuran
Catalog No.:BCN7444
CAS No.:167278-41-3
- Zosuquidar
Catalog No.:BCC2074
CAS No.:167354-41-8
- Fmoc-Lys(Mtt)-OH
Catalog No.:BCC3523
CAS No.:167393-62-6
- H-Cys(Bzl)-OMe.HCl
Catalog No.:BCC2907
CAS No.:16741-80-3
- Sophoracarpan A
Catalog No.:BCN6980
CAS No.:1674359-82-0
- Sophoracarpan B
Catalog No.:BCN6979
CAS No.:1674359-84-2
- LY335979 (Zosuquidar 3HCL)
Catalog No.:BCC3878
CAS No.:167465-36-3
- BADGE
Catalog No.:BCC7022
CAS No.:1675-54-3
- PD-1/PD-L1 inhibitor 2
Catalog No.:BCC6520
CAS No.:1675203-84-5
- 11-Hydroxy-12-methoxyabietatriene
Catalog No.:BCN3253
CAS No.:16755-54-7
Structural Analysis and Quantitative Determination of Clevidipine Butyrate Impurities Using an Advanced RP-HPLC Method.[Pubmed:26489435]
J Chromatogr Sci. 2016 Mar;54(3):353-60.
Eleven potential impurities, including process-related compounds and degradation products, have been analyzed by comprehensive studies on the manufacturing process of Clevidipine Butyrate. Possible formation mechanisms could also be devised. MS and NMR techniques have been used for the structural characterization of three previously unreported impurities (Imp-3, Imp-5 and Imp-11). To separate and quantify the potential impurities in a simultaneous fashion, an efficient and advanced RP-HPLC method has been developed. In doing so, four major degradation products (Imp-2, Imp-4, Imp-8 and Imp-10) can be observed under varying stress conditions. This analytical method has been validated according to ICH guidelines with respect to specificity, accuracy, linearity, robustness and stability. The method described has been demonstrated to be applicable in routine quality control processes and stability evaluation studies of Clevidipine Butyrate.
Role of clevidipine butyrate in the treatment of acute hypertension in the critical care setting: a review.[Pubmed:20730061]
Vasc Health Risk Manag. 2010 Aug 9;6:457-64.
Acutely elevated blood pressure in the critical care setting is associated with a higher risk of acute end-organ damage (eg, myocardial ischemia, stroke, and renal failure) and perioperative bleeding. Urgent treatment and careful blood pressure control are crucial to prevent significant morbidity. Clevidipine Butyrate (Cleviprex) is an ultrashort-acting, third-generation intravenous calcium channel blocker. It is an arterial-selective vasodilator with no venodilatory or myocardial depressive effects. Clevidipine has an extremely short half-life of approximately 1 minute as it is rapidly metabolized by blood and tissue esterases. These metabolites are then primarily eliminated through urine and fecal pathways. The rapid onset and the short duration of action permit tighter and closer adjustment of the blood pressure than is possible with other intravenous agents.
[Simultaneous determination of clevidipine butyrate and its metabolite clevidipine acid in dog blood by liquid chromatography-tandem mass spectrometry].[Pubmed:26837176]
Yao Xue Xue Bao. 2015 Oct;50(10):1290-6.
A rapid, sensitive and simple liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed and validated for the simultaneous determination of Clevidipine Butyrate and its primary metabolite clevidipine acid in dog blood. After one-step protein precipitation with methanol, the chromatographic separation was carried out on an Ecosil C18 column (150 mm x 4.6 mm, 5 microm) with a gradient mobile phase consisting of methanol and 5 mmol . L(-1) ammonium formate. A chromatographic total run time of 13.0 min was achieved. The quantitation analysis was performed using multiple reaction monitoring (MRM) at the specific ion transitions of m/z 454.1 [M-H]- --> m/z 234.1 for Clevidipine Butyrate, m/z 354.0 [M-H]- --> m/z 208.0 for clevidipine acid and m/z 256.1 [M-H]- --> m/z 227.1 for elofesalamide (internal standard, IS) in the negative ion mode with electrospray ionization (ESI) source. The linear calibration curves for Clevidipine Butyrate and clevidipine acid were obtained in the concentration ranges of 0.5-100 ng . mL and 1-200 ng . mL(-1), separately. The lower limit of quantification of Clevidipine Butyrate and clevidipine acid were 0.5 ng . mL(-1) and 1 ng . mL(-1). The intra and inter-assay precisions were all below 12.9%, the accuracies were all in standard ranges. Stability testing indicated that Clevidipine Butyrate and clevidipine acid in dog blood with the addition of denaturant methanol was stable under various processing and/or handling conditions. The validated method has been successfully applied to a pharmacokinetic study of Clevidipine Butyrate injection to 8 healthy Beagle dogs following intravenous infusion at a flow rate of 5 mg . h(-1) for 0.5 h.


