R-96544 hydrochloridePotent, selective 5-HT2A antagonist CAS# 167144-80-1 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 167144-80-1 | SDF | Download SDF |
| PubChem ID | 16759150 | Appearance | Powder |
| Formula | C22H30ClNO3 | M.Wt | 391.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
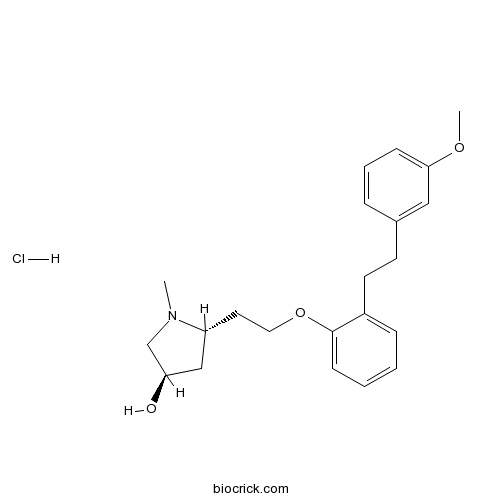
| Chemical Name | (3R,5R)-5-[2-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]ethyl]-1-methylpyrrolidin-3-ol;hydrochloride | ||
| SMILES | CN1CC(CC1CCOC2=CC=CC=C2CCC3=CC(=CC=C3)OC)O.Cl | ||
| Standard InChIKey | OUKRSTJUAQEATQ-GZJHNZOKSA-N | ||
| Standard InChI | InChI=1S/C22H29NO3.ClH/c1-23-16-20(24)15-19(23)12-13-26-22-9-4-3-7-18(22)11-10-17-6-5-8-21(14-17)25-2;/h3-9,14,19-20,24H,10-13,15-16H2,1-2H3;1H/t19-,20-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective 5-HT2 receptor antagonist; displays some selectivity for 5-HT2A receptors (Ki = 1.6 nM). IC50 values are 2.2, 310, 2400, 3700, > 5000 and > 5000 nM for 5-HT2, α1-adrenergic, D2 dopamine, 5-HT1, 5-HT3 and β-adrenergic receptors respectively. Inhibits 5-HT-induced platelet aggregation and pressor responses in vivo. |

R-96544 hydrochloride Dilution Calculator

R-96544 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5514 mL | 12.7571 mL | 25.5141 mL | 51.0282 mL | 63.7853 mL |
| 5 mM | 0.5103 mL | 2.5514 mL | 5.1028 mL | 10.2056 mL | 12.7571 mL |
| 10 mM | 0.2551 mL | 1.2757 mL | 2.5514 mL | 5.1028 mL | 6.3785 mL |
| 50 mM | 0.051 mL | 0.2551 mL | 0.5103 mL | 1.0206 mL | 1.2757 mL |
| 100 mM | 0.0255 mL | 0.1276 mL | 0.2551 mL | 0.5103 mL | 0.6379 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Autocamtide-2-related inhibitory peptide
Catalog No.:BCC7153
CAS No.:167114-91-2
- 10-Oxo Docetaxel
Catalog No.:BCC5409
CAS No.:167074-97-7
- Pitavastatin ethyl ester
Catalog No.:BCC9122
CAS No.:167073-19-0
- Fmoc-D-Cys(Trt)-OH
Catalog No.:BCC3481
CAS No.:167015-11-4
- BMS 453
Catalog No.:BCC7679
CAS No.:166977-43-1
- N-Acetoacetylmorpholine
Catalog No.:BCC9078
CAS No.:16695-54-8
- Tetramethylkaempferol
Catalog No.:BCN8082
CAS No.:16692-52-7
- 3-Amino-5-mercapto-1,2,4-triazole
Catalog No.:BCC8615
CAS No.:16691-43-3
- A-1210477
Catalog No.:BCC6508
CAS No.:1668553-26-1
- Chebulanin
Catalog No.:BCN3261
CAS No.:166833-80-3
- H-D-Orn-OH. HCl
Catalog No.:BCC3004
CAS No.:16682-12-5
- H-Gly-NH2.HCl
Catalog No.:BCC2947
CAS No.:1668-10-6
- Furagin
Catalog No.:BCC1582
CAS No.:1672-88-4
- Clevidipine Butyrate
Catalog No.:BCC4401
CAS No.:167221-71-8
- Cyanidin 3-Sophoroside-5-Glucoside
Catalog No.:BCC8158
CAS No.:16727-02-9
- Malvidin 3,5-Diglucoside
Catalog No.:BCC8206
CAS No.:16727-30-3
- 9-Hydroxyeriobofuran
Catalog No.:BCN7444
CAS No.:167278-41-3
- Zosuquidar
Catalog No.:BCC2074
CAS No.:167354-41-8
- Fmoc-Lys(Mtt)-OH
Catalog No.:BCC3523
CAS No.:167393-62-6
- H-Cys(Bzl)-OMe.HCl
Catalog No.:BCC2907
CAS No.:16741-80-3
- Sophoracarpan A
Catalog No.:BCN6980
CAS No.:1674359-82-0
- Sophoracarpan B
Catalog No.:BCN6979
CAS No.:1674359-84-2
- LY335979 (Zosuquidar 3HCL)
Catalog No.:BCC3878
CAS No.:167465-36-3
- BADGE
Catalog No.:BCC7022
CAS No.:1675-54-3
Effects of R-102444 and its active metabolite R-96544, selective 5-HT2A receptor antagonists, on experimental acute and chronic pancreatitis: Additional evidence for possible involvement of 5-HT2A receptors in the development of experimental pancreatitis.[Pubmed:16183055]
Eur J Pharmacol. 2005 Oct 3;521(1-3):156-63.
The effects of R-102444 ((2R, 4R)-4-lauroyloxy-2-[2-[2-[2-(3-methoxy)phenyl]ethyl]phenoxy]ethyl-1-methylpyrroli dine hydrochloride) and its active metabolite R-96544 ((2R, 4R)-2-[2-[2-[2-(3-methoxy)phenyl]ethyl]phenoxy]ethyl-4-hydroxy-1-methylpyrrolidin e hydrochloride), potent and selective 5-hydroxytryptamine 2A (5-HT2A) receptor antagonists, on development of pancreatitis were investigated in experimental models of acute and chronic pancreatitis. Rat acute pancreatitis was induced by caerulein (20 microg/kg) intraperitoneal injection and by pancreatic duct ligation. In both the models, serum amylase and lipase activities were markedly increased. R-102444 dose-dependently reduced these enzyme activities at a dose range of 10 to 100 mg/kg (p.o.) for the caerulein model and 0.3 to 10 mg/kg (p.o.) for the ligation model. In a mouse model of acute pancreatitis induced by a choline-deficient, ethionine (0.5%)-supplemented diet, subcutaneous administration of R-96544 (10-100 mg/kg, bid) reduced serum amylase activity. Histological analysis showed that R-96544 dose-dependently attenuated pancreatic necrosis, inflammation and vacuolization. The effect of R-102444 was further examined in male Wistar Bonn/Kobori rats (4-9 months of age) which spontaneously show pancreatic fibrosis and parenchymal destruction compatible with human chronic pancreatitis. In Wistar Bonn/Kobori rats (from 3 to 9 months of age) fed a diet containing 0.017% and 0.17% of R-102444, pancreatic weight, pancreatic protein and amylase content were higher compared to those in non-treated pancreatitis control rats. Histological analysis showed that R-102444 suppressed parenchymal destruction and replacement with adipose tissue, indicating inhibition of pancreatic atrophy. These results clearly indicate that R-102444 and R-96544 inhibit the progression of acute and chronic pancreatitis and support the contention of possible involvement of 5-HT2A receptors in the progression of experimental pancreatitis.
Pharmacological profiles of R-96544, the active form of a novel 5-HT2A receptor antagonist R-102444.[Pubmed:12464356]
Eur J Pharmacol. 2002 Dec 20;457(2-3):107-14.
We examined the pharmacology of (2R,4R)-4-hydroxy-2-[2-[2-[2-(3-methoxy)phenyl]ethyl]phenoxy]ethyl-1-methylpyrrol idine hydrochloride (R-96544), the active form of a novel 5-HT(2A) receptor antagonist, (2R,4R)-4-lauroyloxy-2-[2-[2-[2-(3-methoxy)phenyl]ethyl]phenoxy]ethyl-1-methylpyr rolidine hydrochloride (R-102444). R-96544 produced a concentration-dependent inhibition of platelet aggregation induced by serotonin (5-hydroxytryptamine, 5-HT) alone or in combination with ADP in platelets from humans, monkeys, cats, rabbits, rats and mice. An intravenous administration of R-96544 to rabbits significantly inhibited ex vivo platelet aggregation induced by 5-hydroxytryptamine (5-HT) combined with epinephrine. An oral administration of R-102444 to rats also resulted in significant inhibition of ex vivo platelet aggregation, whereas R-102444 was ineffective in an in vitro platelet aggregation assay. These antiplatelet effects of R-96544 and R-102444 were more potent than those of two other 5-HT(2A) receptor antagonists, sarpogrelate and its active metabolite (+/-)-1-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]-3-(dimethylamino)-2-propanol hydrochloride (M-1). A binding study using cat platelet membranes showed that R-96544 has high affinity for 5-HT(2A) receptors but no effect on non-serotonergic [3H]ketanserin-binding sites. R-96544 caused a parallel shift to the right of concentration-response curves for 5-HT in rat caudal artery contraction mediated by 5-HT(2A) receptors. Schild plot analysis gave a pA(2) value of 10.4 with a slope near unity (1.04). R-96544 also inhibited 5-HT(2A) receptor-mediated contraction of guinea pig trachea but not 5-HT(3) receptor-mediated contraction of guinea pig ileum and 5-HT(2B) receptor-mediated contraction of rat fundus preparation. R-96544 (i.v.) attenuated the pressor responses evoked by 5-HT (15 microg/kg, i.v.) but not by phenylephrine (5 microg/kg, i.v.) and angiotensin II (0.1 microg/kg, i.v.), after ganglionic blockade in anesthetized spontaneously hypertensive rats. These results show that R-96544, the active form of R-102444, is a novel 5-HT receptor antagonist with potent, competitive, and 5-HT(2A)-selective activity.
[2-(O-Phenylalkyl)phenoxy]alkylamines III: Synthesis and selective serotonin-2 receptor binding (2).[Pubmed:11086903]
Chem Pharm Bull (Tokyo). 2000 Nov;48(11):1729-39.
A series of 12-(2-phenylethyl)phenoxy]ethylpyrrolidine derivatives were synthesized, and their affinity for serotonin-2 (5-HT2) and dopamine-2 (D2) receptors was examined. Among them, compound 17, (2R,4R)-4-hydroxy-2-[2-[2-[2-(3-methoxyphenyl)ethyl]phenoxylethyl] -1-methylpyrrolidine hydrochloride, showed high 5-HT2 receptor affinity in vitro. This compound was a more potent inhibitor of ex vivo 5-HT-induced platelet aggregation than compound 3, which was previously shown to be more potent than ketanserin (1) and sarpogrelate (2a). However, compound 17 produced gastric irritation in rats. Therefore, we carried out a further derivatization of 17, and compound 45 (R-102444), a lauryl ester prodrug of compound 17, was found to be a promising candidate as an antithrombotic agent. Oral administration of R-102444 produced a marked inhibition of 5-HT-induced ex vivo platelet aggregation, and R-102444 did not cause any gastric irritation. The antiaggregatory effects of R-102444 were more potent than those of sarpogrelate (2a) and its active metabolite, M-1 (2b). In addition, R-102444 exhibited more potent antithrombotic effects than sarpogrelate in a rat photochemically-induced thrombosis model.


