Autocamtide-2-related inhibitory peptideinhibitor of calmodulin-dependent protein kinase II (CaM-kinase II, CaMKII) CAS# 167114-91-2 |
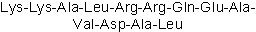
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- SCH-1473759
Catalog No.:BCC1934
CAS No.:1094069-99-4
- Reversine
Catalog No.:BCC1892
CAS No.:656820-32-5
- AZD1152
Catalog No.:BCC1393
CAS No.:722543-31-9
- XL228
Catalog No.:BCC2058
CAS No.:898280-07-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 167114-91-2 | SDF | Download SDF |
| PubChem ID | 126456179 | Appearance | Powder |
| Formula | C64H116N22O19 | M.Wt | 1497.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AIP | ||
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | KKALRRQEAVDAL | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2,6-diaminohexanoyl]amino]hexanoyl]amino]propanoyl]amino]-4-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-oxopentanoyl]amino]-4-carboxybutanoyl]amino]propanoyl]amino]-3-methylbutanoyl]amino]-3-carboxypropanoyl]amino]propanoyl]amino]-4-methylpentanoic acid;2,2,2-trifluoroacetic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=O)O)C(=O)NC(C)C(=O)NC(C(C)C)C(=O)NC(CC(=O)O)C(=O)NC(C)C(=O)NC(CC(C)C)C(=O)O)NC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CCCCN)N.C(=O)(C(F)(F)F)O | ||
| Standard InChIKey | AQERPQDFAQEHFS-PNTPNKCPSA-N | ||
| Standard InChI | InChI=1S/C64H116N22O19.C2HF3O2/c1-31(2)28-43(83-50(92)34(7)75-54(96)38(17-11-13-25-66)78-53(95)37(67)16-10-12-24-65)60(102)80-40(19-15-27-74-64(71)72)56(98)79-39(18-14-26-73-63(69)70)57(99)81-41(20-22-46(68)87)58(100)82-42(21-23-47(88)89)55(97)76-36(9)52(94)86-49(33(5)6)61(103)84-44(30-48(90)91)59(101)77-35(8)51(93)85-45(62(104)105)29-32(3)4;3-2(4,5)1(6)7/h31-45,49H,10-30,65-67H2,1-9H3,(H2,68,87)(H,75,96)(H,76,97)(H,77,101)(H,78,95)(H,79,98)(H,80,102)(H,81,99)(H,82,100)(H,83,92)(H,84,103)(H,85,93)(H,86,94)(H,88,89)(H,90,91)(H,104,105)(H4,69,70,73)(H4,71,72,74);(H,6,7)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,49-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective and potent calmodulin-dependent protein kinase II (CaM kinase II) inhibitor (IC50 = 40 nM). Selective over PKC, PKA and CaM kinase IV (IC50 > 10 μM). |

Autocamtide-2-related inhibitory peptide Dilution Calculator

Autocamtide-2-related inhibitory peptide Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 40 nM for CaM-kinase II [1].
Synthetic peptide AIP (autocamtide-2-related inhibitory peptide) is a nonphosphorylatable analog of autocamtide-2, which was identified to be a highly specific and potent inhibitor of calmodulin-dependent protein kinase II (CaM-kinase II, CaMKII). CaMKII is a serine/threonine-specific protein kinase, which is modulated by the Ca2+/calmodulin.
In vitro: AIP (1 mM) completely inhibited CaMKII activity, but did not affect cAMP-dependent protein kinase, calmodulin-dependent protein kinase IV and protein kinase C,. The inhibition was noncompetitive, and the action was caused by binding to the autophosphorylation site, which is distinc from that for the exogenous substrate. The IC50 for the autophosphorylation of CaM II is 100 nM [1].
In vivo: Mice treated with AIP by transgenic expression of AIP, were protected from fructose-rich diet-induced arrhythmogenesis, spontaneous contractions and spontaneous Ca2+ release events [2]. Intra-nucleus accumbens (NAc) injection of AIP could dose-dependently increase the HWL (hindpaw withdrawal latency) to noxious thermal and mechanical stimulation in rats with mononeuropathy [3].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Ishida A1, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995 Jul 26;212(3):806-12.
[2] Sommese L, Valverde CA, Blanco P, Castro MC, Rueda OV, Kaetzel M, Dedman J, Anderson ME, Mattiazzi A, Palomeque J. Ryanodine receptor phosphorylation by CaMKII promotes spontaneous Ca(2+) release events in a rodent model of early stage diabetes: The arrhythmogenic substrate. Int J Cardiol. 2016 Jan 1;202:394-406.
[3] Bian H, Yu LC. Intra-nucleus accumbens administration of the calcium/calmodulin-dependent protein kinase II inhibitor AIP induced antinociception in rats with mononeuropathy. Neurosci Lett. 2015 Jul 10;599:129-32.
- 10-Oxo Docetaxel
Catalog No.:BCC5409
CAS No.:167074-97-7
- Pitavastatin ethyl ester
Catalog No.:BCC9122
CAS No.:167073-19-0
- Fmoc-D-Cys(Trt)-OH
Catalog No.:BCC3481
CAS No.:167015-11-4
- BMS 453
Catalog No.:BCC7679
CAS No.:166977-43-1
- N-Acetoacetylmorpholine
Catalog No.:BCC9078
CAS No.:16695-54-8
- Tetramethylkaempferol
Catalog No.:BCN8082
CAS No.:16692-52-7
- 3-Amino-5-mercapto-1,2,4-triazole
Catalog No.:BCC8615
CAS No.:16691-43-3
- A-1210477
Catalog No.:BCC6508
CAS No.:1668553-26-1
- Chebulanin
Catalog No.:BCN3261
CAS No.:166833-80-3
- H-D-Orn-OH. HCl
Catalog No.:BCC3004
CAS No.:16682-12-5
- H-Gly-NH2.HCl
Catalog No.:BCC2947
CAS No.:1668-10-6
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
- R-96544 hydrochloride
Catalog No.:BCC7164
CAS No.:167144-80-1
- Furagin
Catalog No.:BCC1582
CAS No.:1672-88-4
- Clevidipine Butyrate
Catalog No.:BCC4401
CAS No.:167221-71-8
- Cyanidin 3-Sophoroside-5-Glucoside
Catalog No.:BCC8158
CAS No.:16727-02-9
- Malvidin 3,5-Diglucoside
Catalog No.:BCC8206
CAS No.:16727-30-3
- 9-Hydroxyeriobofuran
Catalog No.:BCN7444
CAS No.:167278-41-3
- Zosuquidar
Catalog No.:BCC2074
CAS No.:167354-41-8
- Fmoc-Lys(Mtt)-OH
Catalog No.:BCC3523
CAS No.:167393-62-6
- H-Cys(Bzl)-OMe.HCl
Catalog No.:BCC2907
CAS No.:16741-80-3
- Sophoracarpan A
Catalog No.:BCN6980
CAS No.:1674359-82-0
- Sophoracarpan B
Catalog No.:BCN6979
CAS No.:1674359-84-2
- LY335979 (Zosuquidar 3HCL)
Catalog No.:BCC3878
CAS No.:167465-36-3
Transport of a Novel Angiotensin-I-Converting Enzyme Inhibitory Peptide Ala-His-Leu-Leu Across Human Intestinal Epithelial Caco-2 Cells.[Pubmed:28296590]
J Med Food. 2017 Mar;20(3):243-250.
The transport behavior and absorption mechanism of Ala-His-Leu-Leu (AHLL) intestinal absorption in Caco-2 cell monolayers were clarified systemically. The safe absorptive concentration of AHLL was 200 mug/mL, which was determined by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay. The permeation of AHLL was concentration dependent in a bidirectional transfer and reached a plateau at 90 min. The efflux ratio was above 0.5, suggesting that AHLL was absorbed by both active transport and passive diffusion. The apparent permeability coefficients (Papp) of AHLL both from the apical (AP) to basolateral (BL) side (PappAB) and from the BL to AP side (PappBA) decreased when the temperature was lowered from 37 degrees C to 4 degrees C.The uptake of AHLL was more at pH 7.4 than at other pHs. Both verapamil and (E)-3-[[[3-[2-(7-chloro-2- quinolinyl) ethenyl] phenyl]-[[(3-dimethyl amino)-3-oxopropyl]thio] methyl] thio]-propanoic acid (MK571) inhibited the absorption of AHLL, indicating that P-glycoprotein and multi-drug resistant proteins (MRPs) were all involved in AHLL secretion, especially multi-drug resistant protein 2 (MRP2). AHLL was transported through both trans- and paracellular pathways across the Caco-2 cell monolayer. This work first elucidates the AHLL absorption mechanism in Caco-2 cells and provides the basis for future studies on the improvement of bioavailability.
Molecular pathways underlying inhibitory effect of antimicrobial peptide Nal-P-113 on bacteria biofilms formation of Porphyromonas gingivalis W83 by DNA microarray.[Pubmed:28212615]
BMC Microbiol. 2017 Feb 17;17(1):37.
BACKGROUND: Wound-related infection remains a major challenge for health professionals. One disadvantage in conventional antibiotics is their inability to penetrate biofilms, the main protective strategy for bacteria to evade irradiation. Previously, we have shown that synthetic antimicrobial peptides could inhibit bacterial biofilms formation. RESULTS: In this study, we first delineated how Nal-P-113, a novel antimicrobial peptide, exerted its inhibitory effects on Porphyromonas gingivalis W83 biofilms formation at a low concentration. Secondly, we performed gene expression profiling and validated that Nal-P-113 at a low dose significantly down-regulated genes related to mobile and extrachromosomal element functions, transport and binding proteins in Porphyromonas gingivalis W83. CONCLUSIONS: These findings suggest that Nal-P-113 at low dose is sufficient to inhibit the formation of biofilms although Porphyromonas gingivalis W83 may maintain its survival in the oral cavity. The newly discovered molecular pathways may add the knowledge of developing a new strategy to target bacterial infections in combination with current first-line treatment in periodontitis.
A multifunctional alanine-rich anti-inflammatory peptide BCP61 showed potent inhibitory effects by inhibiting both NF-kappaB and MAPK expression.[Pubmed:28214973]
Inflammation. 2017 Apr;40(2):688-696.
The purified BCP61 was reported to be a unique low-molecular-weight (MW) anti-microbial peptide because of its non-identical alanine-rich N-terminal sequence. In this study, we investigated the anti-inflammatory effects of BCP61 on induction of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), pro-inflammatory cytokines, nuclear factor-kappa B (NF-kappaB), and mitogen-activated protein kinases (MAPKs) in lipopolysaccharide (LPS)-stimulated Raw 264.7 cells. The treatment with BCP61, with varying concentrations of 10, 50, and 100 mug/mL, inhibited levels of expression of LPS-induced NF-kappaB and MAPKs (extracellular signal-related kinases (ERKs), c-Jun NH2-terminal kinase (JNK), and mitogen-activated protein (p38)) as well as production of pro-inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor-alpha (TNF-alpha), and interleukin-6 (IL-6). The results suggested that BCP61 prevents inhibitor of kappa B (IkappaBalpha) phosphorylation and degradation, thereby inhibiting the nuclear translocation of the p65 protein. We do report that the use of BCP61 in the treatment of inflammation as well as microbial infection could be a potent therapeutic candidate.
The Potent Inhibitory Effect of beta-D-Mannuronic Acid (M2000) as a Novel NSAID with Immunosuppressive Property on Anti-Cyclic Citrullinated Peptide Antibodies, Rheumatoid Factor and Anti-dsDNA Antibodies in Patients with Rheumatoid Arthritis.[Pubmed:28325148]
Curr Drug Discov Technol. 2017;14(3):206-214.
OBJECTIVE: To investigate the inhibitory effect of beta-D-mannuronic acid (M2000) on anti-cyclic citrullinated peptide antibodies (anti-CCP), rheumatoid factor (RF), antidouble strand DNA (anti-dsDNA) and acute phase reactants in rheumatoid arthritis (RA) patients. METHODS: The study included 40 patients with RA who had an inadequate response to conventional therapy (identifier: IRCT2014011213739N2). The patients were permitted to continue the conventional therapy excluding NSAIDs. 21 of them were treated orally by M2000 at a dose of 500 mg twice daily for 12 weeks and the others did not. Serum samples were collected at baseline, 4 weeks and 12 weeks after treatment and were tested for the serum level of anti-CCP and anti-dsDNA antibodies using enzyme linked immunosorbent assay. The serum level of RF and C-reactive proteins (CRP) was determined by the immunoturbidimetric assay, respectively. RESULTS: At baseline, all patients in the M2000 treated group and the control group were positive for anti-CCP, RF. moreover, 4 of 21 (19%) in the M2000 treated group and 2 of the 19 (10.5%) patients in the control group were positive for anti-dsDNA antibodies, respectively. The serum levels of anti-CCP, RF and anti-dsDNA were decreased significantly after M2000 therapy (p<0.001, p<0.001 and p<0.001, respectively). The reduction in the level of anti-CCP was positively correlated with disease activity, swollen joint count and CRP. Furthermore, the level of inflammatory markers ESR and CRP decreased significantly after M2000 therapy (p<0.001 and p<0.004, respectively). CONCLUSION: M2000 shows inhibitory effect on anti-CCP, RF, anti-dsDNA antibodies and acute phase reactants in RA patients.
A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II.[Pubmed:7626114]
Biochem Biophys Res Commun. 1995 Jul 26;212(3):806-12.
A novel synthetic peptide AIP (Autocamtide-2-related inhibitory peptide), a nonphosphorylatable analog of autocamtide-2, was found to be a highly specific and potent inhibitor of calmodulin-dependent protein kinase II (CaM-kinase II). It was 50 and 500 times more potent than CaMK-(281-302Ala286) and KN-93, respectively, under the assay conditions used. The inhibition was unaffected by the presence or absence of Ca2+/calmodulin, and it was competitive with autocamtide-2 and noncompetitive with syntide-2. AIP (1 microM) completely inhibited CaM-kinase II activity, but did not affect cyclic AMP-dependent protein kinase, protein kinase C, calmodulin-dependent protein kinase IV, and unidentified protein kinases occurring in a rat brain extract. These results indicate that AIP is a useful tool for studying the physiological roles of CaM-kinase II.
Stabilization of calmodulin-dependent protein kinase II through the autoinhibitory domain.[Pubmed:7836445]
J Biol Chem. 1995 Feb 3;270(5):2163-70.
The active 30-kDa chymotryptic fragment of calmodulin-dependent protein kinase II (CaM kinase II), devoid of the autoinhibitory domain, and the enzyme, autothiophosphorylated at Thr286/Thr287, were much more labile than was the original native enzyme. They were markedly stabilized by synthetic peptides, designed after the sequence around the autophosphorylation site in the autoinhibitory domain, such as autocamtide-2 and CaMK-(281-309), but such marked stabilizations were not observed with the ordinary exogenous substrates, such as syntide-2. These results suggest that the autoinhibitory domain of CaM kinase II plays a crucial role in stabilizing the enzyme. A nonphosphorylatable analog of autocamtide-2, AIP, strongly inhibited the activity of the 30-kDa fragment. Kinetic analysis revealed that the inhibition by AIP was competitive with respect to autocamtide-2 and CaMK-(281-289) and noncompetitive with respect to syntide-2 and ATP/Mg2+, suggesting that CaM kinase II possesses at least two distinct substrate-binding sites; one for ordinary exogenous substrates such as syntide-2 and the other for an endogenous substrate, the autophosphorylation site (Thr286/Thr287) in the autoinhibitory domain. Fluorescence analysis of the binding of 7-nitrobenz-2-oxa-1,3-diazole-4-yl labeled AIP to the 30-kDa fragment also supported this contention. Thus, the autoinhibitory domain appears to play a crucial role in keeping the enzyme stable by binding to the substrate-binding site for the autophosphorylation site.
Requirement of calmodulin-dependent protein kinase II in cyclic ADP-ribose-mediated intracellular Ca2+ mobilization.[Pubmed:8530441]
J Biol Chem. 1995 Dec 22;270(51):30257-9.
Cyclic ADP-ribose (cADPR) is generated in pancreatic islets by glucose stimulation, serving as a second messenger for Ca2+ mobilization from the endoplasmic reticulum for insulin secretion (Takasawa, S., Nata, K., Yonekura, H., and Okamoto, H. (1993) Science 259, 370-373). In the present study, we observed that the addition of calmodulin (CaM) to rat islet microsomes sensitized and activated the cADPR-mediated Ca2+ release. Inhibitors for CaM-dependent protein kinase II (CaM kinase II) completely abolished the glucose-induced insulin secretion as well as the cADPR-mediated and CaM-activated Ca2+ mobilization. Western blot analysis revealed that the microsomes contain the alpha isoform of CaM kinase II but do not contain CaM. When the active 30-kDa chymotryptic fragment of CaM kinase II was added to the microsomes, fully activated cADPR-mediated Ca2+ release was observed in the absence of CaM. These results along with available evidence strongly suggest that CaM kinase II is required to phosphorylate and activate the ryanodine-like receptor, a Ca2+ channel for cADPR as an endogenous activator, for the cADPR-mediated Ca2+ release.


