BisabolangeloneCAS# 30557-81-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30557-81-4 | SDF | Download SDF |
| PubChem ID | 12300142 | Appearance | Powder |
| Formula | C15H20O3 | M.Wt | 248.32 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
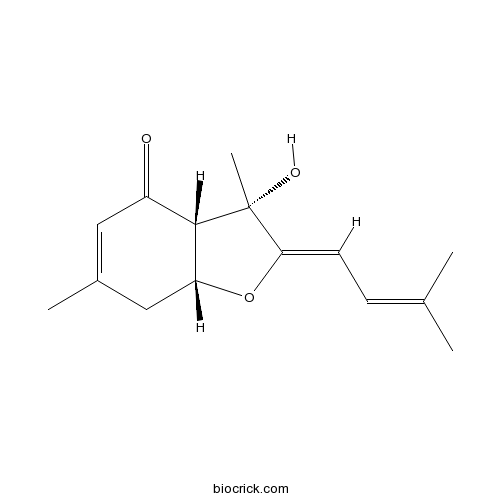
| Chemical Name | (2Z,3S,3aS,7aR)-3-hydroxy-3,6-dimethyl-2-(3-methylbut-2-enylidene)-7,7a-dihydro-3aH-1-benzofuran-4-one | ||
| SMILES | CC1=CC(=O)C2C(C1)OC(=CC=C(C)C)C2(C)O | ||
| Standard InChIKey | GNWNPLBSEQDDQV-FRPWFYLFSA-N | ||
| Standard InChI | InChI=1S/C15H20O3/c1-9(2)5-6-13-15(4,17)14-11(16)7-10(3)8-12(14)18-13/h5-7,12,14,17H,8H2,1-4H3/b13-6-/t12-,14+,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Bisabolangelone has anti-tumor, anti-microbial, and antioxidant activities. 2. Bisabolangelone has anti-inflammatory activity, it inhibits dendritic cell functions by blocking MAPK and NF-κB signaling. 3. Bisabolangelone has antimelanogenic activity, the cAMP-binding site of PKA as a putative target ameliorating melanocyte-specific hyperpigmented disorder. 4. Bisabolangelone has inhibitory effects of the melanogenesis against α-melanocyte stimulating hormone (α-MSH)-activated B16 melanoma cells. 5. Bisabolangelone shows minor activity at the GABA(A) receptor. 6. Bisabolangelone has anti-ulcer activity, it is possible that bisabolangelone inhibited the activity of the H(+)/K(+)-ATPase, then reducing the secretion of H(+). 7. Bisabolangelone inhibits the activity of 5alpha-reductase type I in LNCaP cells (IC50 = 11.6 microg/ml). |
| Targets | TNF-α | IL Receptor | MAPK | NF-kB | p65 | cAMP | PKA | GABA Receptor | NO | PGE | ATPase | Potassium Channel |

Bisabolangelone Dilution Calculator

Bisabolangelone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0271 mL | 20.1353 mL | 40.2706 mL | 80.5412 mL | 100.6765 mL |
| 5 mM | 0.8054 mL | 4.0271 mL | 8.0541 mL | 16.1082 mL | 20.1353 mL |
| 10 mM | 0.4027 mL | 2.0135 mL | 4.0271 mL | 8.0541 mL | 10.0677 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8054 mL | 1.6108 mL | 2.0135 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8054 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Etofenamate
Catalog No.:BCC1563
CAS No.:30544-47-9
- H-Cys(Bzl)-OH
Catalog No.:BCC2906
CAS No.:3054-01-1
- 5'-Demethylaquillochin
Catalog No.:BCN5221
CAS No.:305364-91-4
- Caulilexin C
Catalog No.:BCN3960
CAS No.:30536-48-2
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
- Licoricidin
Catalog No.:BCN6679
CAS No.:30508-27-1
- L-carnosine
Catalog No.:BCN3803
CAS No.:305-84-0
- Chlorambucil
Catalog No.:BCC5351
CAS No.:305-03-3
- 6,7-Dihydroxycoumarin
Catalog No.:BCN5905
CAS No.:305-01-1
- SANT-1
Catalog No.:BCC3941
CAS No.:304909-07-7
- AGK 2
Catalog No.:BCC7609
CAS No.:304896-28-4
- Stavudine (d4T)
Catalog No.:BCC5028
CAS No.:3056-17-5
- Acephate
Catalog No.:BCC7555
CAS No.:30560-19-1
- Geldanamycin
Catalog No.:BCC2125
CAS No.:30562-34-6
- H-Asp(OtBu)-OH
Catalog No.:BCC2891
CAS No.:3057-74-7
- 3,9-Bis(2-cyanoethyl)-2,4,8,10-tetraoxaspiro[5.5]undecane
Catalog No.:BCC8599
CAS No.:3058-04-6
- SC 79
Catalog No.:BCC6246
CAS No.:305834-79-1
- Homovanillic acid
Catalog No.:BCN1253
CAS No.:306-08-1
- Spermine tetrahydrochloride
Catalog No.:BCC6864
CAS No.:306-67-2
- 2,2-Diphenylglycine
Catalog No.:BCC8496
CAS No.:3060-50-2
- AVE 0991 sodium salt
Catalog No.:BCC4222
CAS No.:306288-04-0
- (20S)-Protopanaxdiol
Catalog No.:BCN1254
CAS No.:30636-90-9
- Cycloolivil
Catalog No.:BCN4081
CAS No.:3064-05-9
Bisabolangelone isolated from Ostericum koreanum inhibits the production of inflammatory mediators by down-regulation of NF-kappaB and ERK MAP kinase activity in LPS-stimulated RAW264.7 cells.[Pubmed:19879381]
Int Immunopharmacol. 2010 Feb;10(2):155-62.
Bisabolangelone, a sesquiterpene derivative, was isolated from the roots of Osterici Radix (Ostericum koreanum Maximowicz). In this study, the anti-inflammatory effect of Bisabolangelone was investigated to address potential therapeutic effects in lipopolysaccharide (LPS)-stimulated mouse macrophage RAW 264.7 cells. Bisabolangelone significantly inhibited NO, PGE(2), and pro-inflammatory cytokines by suppressing the mRNA and protein expressions of iNOS and COX-2. Bisabolangelone also inhibited the productions of pro-inflammatory cytokines (TNF-alpha, IL-1beta and IL-6) by suppressing the cytokine mRNA and protein expressions. The molecular mechanism of Bisabolangelone-mediated attenuation in RAW 264.7 cells has a close relationship to suppressing the translocation of nuclear factor-kappaB (NF-kappaB) p65 subunit into the nucleus and the phosphorylation of mitogen-activated protein kinases (MAPKs). These results indicate that Bisabolangelone inhibits LPS-stimulated inflammation through the blocking of NF-kappaB and MAPK pathways in macrophages, and demonstrated that Bisabolangelone possesses anti-inflammatory properties.
Bisabolangelone inhibits dendritic cell functions by blocking MAPK and NF-kappaB signaling.[Pubmed:23727177]
Food Chem Toxicol. 2013 Sep;59:26-33.
Bisabolangelone (BISA), isolated from the roots of Angelica koreana, has many pharmacological activities, such as anti-tumor, anti-microbial, antioxidant, and anti-inflammatory activities. In this study, we investigated the anti-inflammatory mechanisms of BISA in dendritic cells (DCs), which play an essential role in innate and adaptive immune responses. BISA attenuated the production of pro-inflammatory cytokines including interleukin (IL)-12, IL-1beta, and tumor necrosis factor-alpha (TNF-alpha), migration to macrophage inflammatory protein-3 beta, and allo-T cell activating ability of DCs. In addition, BISA affected endocytosis of DCs. Molecular studies showed that BISA suppressed MAPK phosphorylation and nuclear translocation of NF-kappaB p50/p65. Taken together, our data suggest that BISA inhibited DC functions by blocking MAPK and NF-kappaB signaling.
The anti-ulcer activities of bisabolangelone from Angelica polymorpha.[Pubmed:19429382]
J Ethnopharmacol. 2009 Jun 22;123(2):343-6.
AIM OF THE STUDY: Evaluate the anti-ulcer effects of Bisabolangelone from Angelica polymorpha Maxim and provide the basic data to further study for the Angelica polymorpha and Bisabolangelone. MATERIALS AND METHODS: Bisabolangelone was isolated from Angelica polymorpha Maxim collected from Shennongjia Forest District of China. The structure of Bisabolangelone was elucidated by NMR and MS spectrums. The anti-ulcer effects were evaluated with length of lesion (mm) and activity of H(+)/K(+)-ATPase in two models induced by ethanol and Pylorus ligation. Experimental groups were administered with different doses of Bisabolangelone (3.8, 7.6 and 15.3 mg/kg). The positive control group was administered omeprazole with a dose of 3.3 mg/kg. RESULTS: Bisabolangelone significantly reduced the length of lesion (3.8, 7.6 and 15.3 mg/kg, P<0.01), inhibited the activity of H(+)/K(+)-ATPase (3.8, 7.6 and 15.3 mg/kg, P<0.01), decreased the volume of gastric juice (7.6 and 15.3 mg/kg, P<0.05), and increased the pH value of gastric juice (7.6 and 15.3 mg/kg, P<0.01, 3.8 mg/kg, P<0.05). CONCLUSIONS: Bisabolangelone is the main anti-ulcer active compound of Angelica polymorpha, and remarkably preventive and therapeutic action on gastric ulcer. It is possible that Bisabolangelone inhibited the activity of the H(+)/K(+)-ATPase, then reducing the secretion of H(+), and the anti-ulcer mechanism of Bisabolangelone was deserved to be further studied.
Melanogenesis inhibitory bisabolane-type sesquiterpenoids from the roots of Angelica koreana.[Pubmed:22450129]
Bioorg Med Chem Lett. 2012 Apr 15;22(8):2927-31.
Bioactivity-guided isolation of the methanolic extract of the roots of Angelica koreana led to the isolation of four new bisabolane-type sesquiterpenoids, osterivolones A-D (1-4) together with four known compounds, Bisabolangelone (5), decursinol angelate (6), psoralen (7), and falcarindiol (8). Their structures were elucidated on the basis of spectroscopic data interpretation, especially 2D NMR spectra such as HMQC, HMBC, and NOESY. All compounds were evaluated for their inhibitory effects of the melanogenesis against alpha-melanocyte stimulating hormone (alpha-MSH)-activated B16 melanoma cells.
HPLC-based activity profiling of Angelica pubescens roots for new positive GABAA receptor modulators in Xenopus oocytes.[Pubmed:21147202]
Fitoterapia. 2011 Apr;82(3):434-40.
A petroleum ether extract of the traditional Chinese herbal drug Duhuo (roots of Angelica pubescens Maxim. f. biserrata Shan et Yuan), showed significant activity in a functional two-microelectrode voltage clamp assay with Xenopus oocytes which expressed recombinant gamma-aminobutyric acid type A (GABA(A)) receptors of the subtype alpha(1)beta(2)gamma(2S). HPLC-based activity profiling of the active extract revealed six compounds responsible for the GABA(A) receptor modulating activity. They were identified by microprobe NMR and high resolution mass spectrometry as columbianetin acetate (1), imperatorin (3), cnidilin (4), osthol (5), and columbianedin (6). In concentration-dependent experiments, osthol and cnidilin showed the highest potentiation of the GABA induced chloride current (273.6%+/-39.4% and 204.5%+/-33.2%, respectively at 300 muM). Bisabolangelone (2) only showed minor activity at the GABA(A) receptor. The example demonstrates that HPLC-based activity profiling is a simple and efficient method to rapidly identify GABA(A) receptor modulators in a bioactive plant extract.
cAMP-binding site of PKA as a molecular target of bisabolangelone against melanocyte-specific hyperpigmented disorder.[Pubmed:23254773]
J Invest Dermatol. 2013 Apr;133(4):1072-9.
Microphthalmia-associated transcription factor (MITF) is inducible in response to cAMP and has a pivotal role in the melanocyte-specific expression of tyrosinase for skin pigmentation. Here we suggest that the cAMP-binding site of protein kinase A (PKA) is a target in the inhibition of the melanogenic process in melanocytes, as evidenced from the molecular mechanism of small molecules such as Bisabolangelone (BISA) and Rp-adenosine 3',5'-cyclic monophosphorothioate (Rp-cAMPS). BISA is a sesquiterpene constituent of Angelica koreana, a plant of the Umbelliferae family, whose roots are used as an alternative medicine. BISA competitively inhibited cAMP binding to the regulatory subunit of PKA and fitted into the cAMP-binding site on the crystal structure of PKA under the most energetically favorable simulation. In alpha-melanocyte-stimulating hormone (alpha-MSH)-activated melanocytes, BISA and Rp-cAMPS nullified cAMP-dependent PKA activation, dissociating catalytic subunits from an inactive holoenzyme complex. They resultantly inhibited cellular phosphorylation of the cAMP-responsive element-binding protein (CREB) or another transcription factor SOX9, thus downregulating the expression of MITF or the tyrosinase gene with decreased melanin production. Taken together, this study defined the antimelanogenic mechanism of BISA or Rp-cAMPS with a notable implication of the cAMP-binding site of PKA as a putative target ameliorating melanocyte-specific hyperpigmented disorder.
Hypopigmenting activity of bisabolangelone isolated from Angelica koreana Maxim. in alpha-melanocyte stimulating hormone-activated B16 or melan-a cells.[Pubmed:20814852]
Planta Med. 2011 Feb;77(3):248-51.
Tyrosinase is a key enzyme in the biosynthetic pathway of melanin pigments. Abnormal accumulation of melanin pigments causes melasma, freckles, and senile lentigo, which can be substantially ameliorated by treatment with arbutin or other tyrosinase inhibitors. In this study, roots of Angelica koreana Maxim. (Umbelliferae) inhibited melanin production in alpha-melanocyte stimulating hormone ( alpha-MSH)-activated B16 melanoma cells or melan-a melanocytes. To elucidate the hypopigmenting principle of A. koreana, the plant extracts were subjected to bioassay-guided phytochemical analysis, resulting in the identification of Bisabolangelone. Bisabolangelone dose-dependently inhibited alpha-MSH-induced melanin production in B16 or melan-a cells with IC(15) values of 9-17 microM. The positive control arbutin also inhibited melanin production in B16 cells with an IC(50) value of 317 microM. Bisabolangelone suppressed alpha-MSH-inducible protein levels of tyrosinase in B16 cells but could not significantly inhibit the catalytic activity of cell-free tyrosinase. Taken together, this study indicates that Bisabolangelone is the primary hypopigmenting principle of A. koreana and may have pharmacological potential in the melanin-associated hyperpigmentation disorders.


