CHIR-090Potent LpxC inhibitor CAS# 728865-23-4 |
- BAY 87-2243
Catalog No.:BCC4131
CAS No.:1227158-85-1
- FG-4592 (ASP1517)
Catalog No.:BCC2227
CAS No.:808118-40-3
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 728865-23-4 | SDF | Download SDF |
| PubChem ID | 11546620 | Appearance | Powder |
| Formula | C24H27N3O5 | M.Wt | 437.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 5 mg/mL (11.43 mM; Need ultrasonic) | ||
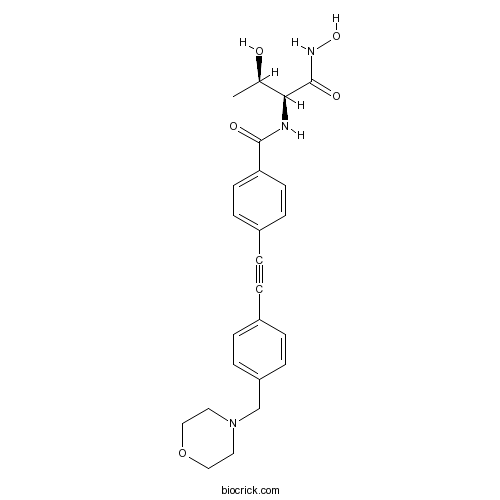
| Chemical Name | N-[(2S,3R)-3-hydroxy-1-(hydroxyamino)-1-oxobutan-2-yl]-4-[2-[4-(morpholin-4-ylmethyl)phenyl]ethynyl]benzamide | ||
| SMILES | CC(C(C(=O)NO)NC(=O)C1=CC=C(C=C1)C#CC2=CC=C(C=C2)CN3CCOCC3)O | ||
| Standard InChIKey | FQYBTYFKOHPWQT-VGSWGCGISA-N | ||
| Standard InChI | InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CHIR-090 is a potent inhibitor of LpxC | |||||
| Targets | LpxC | bacterial | ||||

CHIR-090 Dilution Calculator

CHIR-090 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2858 mL | 11.4288 mL | 22.8577 mL | 45.7153 mL | 57.1442 mL |
| 5 mM | 0.4572 mL | 2.2858 mL | 4.5715 mL | 9.1431 mL | 11.4288 mL |
| 10 mM | 0.2286 mL | 1.1429 mL | 2.2858 mL | 4.5715 mL | 5.7144 mL |
| 50 mM | 0.0457 mL | 0.2286 mL | 0.4572 mL | 0.9143 mL | 1.1429 mL |
| 100 mM | 0.0229 mL | 0.1143 mL | 0.2286 mL | 0.4572 mL | 0.5714 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CHIR-090 is a very potent, low, tight-binding inhibitor of LpxC with Ki value of 4.0 nM [1].
LpxC is a zinc-dependent amidase and present in almost all Gram-negative bacteria. LpxC is a promising target for the development of novel antibiotic substances against multigrug-resistant Gram-negative bacteria [2].
CHIR-090 is a potent LpxC inhibitor and has a different selectivity with the reported LpxC inhibitor L-161. When tested with Escherichia coli LpxC, administration of CHIR-090 showed tight inhibition with Ki value of 4.0 nM, Ki*=0.5 nM, K5=1.9/min and K6=0.18/min [1]. In bacterial P.aeruginosa efflux pupm mutants, CHIR-090 treatment showed inhibition function on MexAB-Oprm, MexCD-OprJ and MexEF-OprN [2]. CHIR-090 showed remarkable antibiotic activity against both E.coli and P.aeruginosa by inhibiting LpxC orthologs at low nM concentrations [3].
In E.coli W3110RL with R.legumunosarum lpxC replacement of E.coli lpxC, CHIR-090 (1 to 10 μg/ml) treatment had no effect on strain growth on LB agar plates while wild-type cells stopped growing after about 2 h in the presence of 1 μg/ml CHIR-090 [1].
References:
[1].Barb, A.W., et al., Inhibition of lipid A biosynthesis as the primary mechanism of CHIR-090 antibiotic activity in Escherichia coli. Biochemistry, 2007. 46(12): p. 3793-802.
[2].Barb, A.W. and P. Zhou, Mechanism and inhibition of LpxC: an essential zinc-dependent deacetylase of bacterial lipid A synthesis. Curr Pharm Biotechnol, 2008. 9(1): p. 9-15.
[3].McClerren, A.L., et al., A slow, tight-binding inhibitor of the zinc-dependent deacetylase LpxC of lipid A biosynthesis with antibiotic activity comparable to ciprofloxacin. Biochemistry, 2005. 44(50): p. 16574-83.
- Agatharesinol
Catalog No.:BCN4594
CAS No.:7288-11-1
- Crocandine
Catalog No.:BCN2070
CAS No.:72855-83-5
- Echitoveniline
Catalog No.:BCN7490
CAS No.:72855-79-9
- 1-Benzyl-5-phenylbarbituric acid
Catalog No.:BCC8461
CAS No.:72846-00-5
- MIRA-1
Catalog No.:BCC2409
CAS No.:72835-26-8
- Deoxyartemisinin
Catalog No.:BCN4285
CAS No.:72826-63-2
- Atanine
Catalog No.:BCN3317
CAS No.:7282-19-1
- OSI-930
Catalog No.:BCC1253
CAS No.:728033-96-3
- Panaxydol
Catalog No.:BCN3701
CAS No.:72800-72-7
- Estropipate
Catalog No.:BCC7719
CAS No.:7280-37-7
- Linderalactone
Catalog No.:BCN1251
CAS No.:728-61-0
- ICI 118,551 hydrochloride
Catalog No.:BCC4029
CAS No.:72795-01-8
- Benzoyloxypeoniflorin
Catalog No.:BCN2799
CAS No.:72896-40-3
- 4-Propenylbrenzcatechin
Catalog No.:BCN3672
CAS No.:72898-29-4
- Isocrocandine
Catalog No.:BCN2071
CAS No.:72903-70-9
- O-Methylcedrelopsin
Catalog No.:BCN3637
CAS No.:72916-61-1
- (Z)-Butylidenephthalide
Catalog No.:BCN4007
CAS No.:72917-31-8
- α,β-Methyleneadenosine 5'-triphosphate trisodium salt
Catalog No.:BCC3591
CAS No.:7292-42-4
- (RS)-4-Carboxyphenylglycine
Catalog No.:BCC6601
CAS No.:7292-81-1
- Bohemamine
Catalog No.:BCN1958
CAS No.:72926-12-6
- K858
Catalog No.:BCC7760
CAS No.:72926-24-0
- Octadecyl p-coumarate
Catalog No.:BCN7235
CAS No.:72943-88-5
- 30-Hydroxylup-20(29)-en-3-one
Catalog No.:BCN4286
CAS No.:72944-06-0
- DL-Catechin
Catalog No.:BCN6325
CAS No.:7295-85-4
Structure of the metal-dependent deacetylase LpxC from Yersinia enterocolitica complexed with the potent inhibitor CHIR-090 .[Pubmed:21171638]
Biochemistry. 2011 Jan 18;50(2):258-65.
The first committed step of lipid A biosynthesis is catalyzed by UDP-(3-O-((R)-3-hydroxymyristoyl))-N-acetylglucosamine deacetylase, a metal-dependent deacetylase also known as LpxC. Because lipid A is essential for bacterial viability, the inhibition of LpxC is an appealing therapeutic strategy for the treatment of Gram-negative bacterial infections. Here we report the 1.79 A resolution X-ray crystal structure of LpxC from Yersinia enterocolitica (YeLpxC) complexed with the potent hydroxamate inhibitor CHIR-090. This enzyme is a nearly identical orthologue of LpxC from Yersinia pestis (99.7% sequence identity), the pathogen that causes bubonic plague. Similar to the inhibition of LpxC from Escherichia coli, CHIR-090 inhibits YeLpxC via a two-step slow, tight-binding mechanism with an apparent K(i) of 0.54 +/- 0.14 nM followed by conversion of the E.I to E.I* species with a rate constant of 0.11 +/- 0.01 min(-1). The structure of the LpxC complex with CHIR-090 shows that the inhibitor hydroxamate group chelates the active site zinc ion, and the "tail" of the inhibitor binds in the hydrophobic tunnel in the active site. This hydrophobic tunnel is framed by a betaalphabeta subdomain that exhibits significant conformational flexibility as it accommodates inhibitor binding. CHIR-090 displays a 27 mm zone of inhibition against Y. enterocolitica in a Kirby-Bauer antibiotic assay, which is comparable to its reported activity against other Gram-negative species including E. coli and Pseudomonas aeruginosa. This study demonstrates that the inhibition of LpxC should be explored as a potential therapeutic and/or prophylatic response to infection by weaponized Yersinia species.
Design and stereoselective synthesis of a C-aryl furanoside as a conformationally constrained CHIR-090 analogue.[Pubmed:22925765]
Carbohydr Res. 2012 Oct 1;359:59-64.
The UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine deacetylase (LpxC) is a promising target for the development of novel antibiotic substances against multidrug-resistant Gram-negative bacteria. The C-aryl glycoside 3 was designed as conformationally constrained analogue of the potent LpxC-inhibitor CHIR-090. The chiral pool synthesis of 3 started with D-mannose. The C-aryl glycoside 8 was synthesized stereoselectively by nucleophilic attack of 4-iodine-substituted phenyllithium and subsequent reduction with Et(3)SiH. The ester 10 was obtained in a one-pot diol cleavage, CrO(3) oxidation, and esterification. A Sonogashira reaction of the aryl iodide 11 led to the alkyne 17 which was transformed with H(2)NOH into the hydroxamic acid 3.
Mechanisms decreasing in vitro susceptibility to the LpxC inhibitor CHIR-090 in the gram-negative pathogen Pseudomonas aeruginosa.[Pubmed:22024823]
Antimicrob Agents Chemother. 2012 Jan;56(1):17-27.
Testing P. aeruginosa efflux pump mutants showed that the LpxC inhibitor CHIR-090 is a substrate for MexAB-OprM, MexCD-OprJ, and MexEF-OprN. Utilizing P. aeruginosa PAO1 with a chromosomal mexC::luxCDABE fusion, luminescent mutants arose on medium containing 4 mug/ml CHIR-090, indicating upregulation of MexCD-OprJ. These mutants were less susceptible to CHIR-090 (MIC, 4 mug/ml) and had mutations in the mexCD-oprJ repressor gene nfxB. Nonluminescent mutants (MIC, 4 mug/ml) that had mutations in the mexAB-oprM regulator gene mexR were also observed. Plating the clinical isolate K2153 on 4 mug/ml CHIR-090 selected mutants with alterations in mexS (immediately upstream of mexT), which upregulates MexEF-OprN. A mutant altered in the putative1ribosomal binding site (RBS) upstream of lpxC and overexpressing LpxC was selected on a related LpxC inhibitor and exhibited reduced susceptibility to CHIR-090. Overexpression of LpxC from a plasmid reduced susceptibility to CHIR-090, and introduction of the altered RBS in this construct further increased expression of LpxC and decreased susceptibility to CHIR-090. Using a mutS (hypermutator) strain, a mutant with an altered lpxC target gene (LpxC L18V) was also selected. Purified LpxC L18V had activity similar to that of wild-type LpxC in an in vitro assay but had reduced inhibition by CHIR-090. Finally, an additional class of mutant, typified by an extreme growth defect, was identified. These mutants had mutations in fabG, indicating that alteration in fatty acid synthesis conferred resistance to LpxC inhibitors. Passaging experiments showed progressive decreases in susceptibility to CHIR-090. Therefore, P. aeruginosa can employ several strategies to reduce susceptibility to CHIR-090 in vitro.


