CinnamolideCAS# 23599-47-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23599-47-5 | SDF | Download SDF |
| PubChem ID | 12303261 | Appearance | Powder |
| Formula | C15H22O2 | M.Wt | 234.33 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
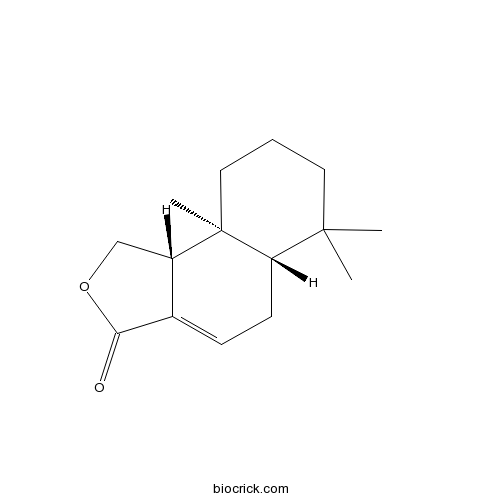
| Chemical Name | (5aS,9aS,9bR)-6,6,9a-trimethyl-5,5a,7,8,9,9b-hexahydro-1H-benzo[e][2]benzofuran-3-one | ||
| SMILES | CC1(CCCC2(C1CC=C3C2COC3=O)C)C | ||
| Standard InChIKey | UMUMRNRVJNFLPT-SLEUVZQESA-N | ||
| Standard InChI | InChI=1S/C15H22O2/c1-14(2)7-4-8-15(3)11-9-17-13(16)10(11)5-6-12(14)15/h5,11-12H,4,6-9H2,1-3H3/t11-,12-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cinnamolide Dilution Calculator

Cinnamolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2675 mL | 21.3374 mL | 42.6749 mL | 85.3497 mL | 106.6872 mL |
| 5 mM | 0.8535 mL | 4.2675 mL | 8.535 mL | 17.0699 mL | 21.3374 mL |
| 10 mM | 0.4267 mL | 2.1337 mL | 4.2675 mL | 8.535 mL | 10.6687 mL |
| 50 mM | 0.0853 mL | 0.4267 mL | 0.8535 mL | 1.707 mL | 2.1337 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4267 mL | 0.8535 mL | 1.0669 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Machigline
Catalog No.:BCN9276
CAS No.:87264-30-0
- Effususol A
Catalog No.:BCN9275
CAS No.:1869082-58-5
- Phoyunbene B
Catalog No.:BCN9274
CAS No.:886747-62-2
- 3-(3-Hydroxybutyl)phenol
Catalog No.:BCN9273
CAS No.:854464-95-2
- 6-Methoxyspirotryprostatin B
Catalog No.:BCN9272
CAS No.:1031727-28-2
- Pregn-5-ene-3β,17α,20S-triol
Catalog No.:BCN9271
CAS No.:903-67-3
- Hedyotol D
Catalog No.:BCN9270
CAS No.:97465-80-0
- Cavaol E
Catalog No.:BCN9269
CAS No.:1233044-20-6
- Platyphyllonol 5-O-β-D-xylopyranoside
Catalog No.:BCN9268
CAS No.:288141-04-8
- Tatsinine
Catalog No.:BCN9267
CAS No.:90038-21-4
- 4-Demethyltraxillaside
Catalog No.:BCN9266
CAS No.:1691201-82-7
- Sepiumol E
Catalog No.:BCN9265
CAS No.:2412027-09-7
- Cassamedine
Catalog No.:BCN9278
CAS No.:16408-75-6
- Integracin A
Catalog No.:BCN9279
CAS No.:224186-03-2
- 2,7-Dihydroxy-1,6-dimethylpyrene
Catalog No.:BCN9280
CAS No.:468103-76-6
- 9,10-Didehydroeffususol A
Catalog No.:BCN9281
CAS No.:1869082-57-4
- Juncuenin D
Catalog No.:BCN9282
CAS No.:1161681-24-8
- N1,N5,N10-Tri-p-coumaroylspermidine
Catalog No.:BCN9283
CAS No.:131086-78-7
- 1-O-p-Coumaroylglycerol
Catalog No.:BCN9284
CAS No.:106055-11-2
- Phoyunbene C
Catalog No.:BCN9285
CAS No.:886747-63-3
- Palmitone
Catalog No.:BCN9286
CAS No.:502-73-8
- Oregonin
Catalog No.:BCN9287
CAS No.:55303-93-0
- Browniine
Catalog No.:BCN9288
CAS No.:5140-42-1
- Rubanthrone A
Catalog No.:BCN9289
CAS No.:441764-20-1
Cinnamolide sesquiterpene lactone suppresses in vitro and in vivo cancer cell growth in cisplatin-resistant human cervical carcinoma cells by inducing mitochondrial mediated apoptosis, caspase activation, loss of MMP and targeting Akt/beta-Catenin signaling pathway.[Pubmed:32521857]
J BUON. 2020 Mar-Apr;25(2):709-715.
PURPOSE: This study was designed to examine the in vitro and in vivo antitumor effects of Cinnamolide against cisplatin-resistant human cervical cancer cells (HeLa cells). METHODS: Cell viability was examined by WST-1 cell viability assay. Cinnamolide-induced apoptosis was examined by fluorescent microscopy using acridine orange (AlphaOmicron) /ethidium bromide (EB) staining and flow cytometry in combination with annexin-V/propidium iodide (PI) staining. Western blot was used to study the effects of Cinnamolide on apoptosis-related protein expressions including Bax and Bcl-2 as well as to study effects on numerous caspases and Akt/beta-Catenin signaling pathway. Effects on mitochondrial membrane potential (MMP) were evaluated by flow cytometry. In vivo studies using xenograft mouse model were carried out to evaluate the efficacy of Cinnamolide under in vivo conditions. RESULTS: Cinnamolide decreased the viability of the HeLa human cervical cancer cells and exhibited an IC50 of 16.5 microM. The cytoxicity of Cinnamolide was also investigated on the MDCK normal cervical cells which showed that Cinnamolide exerted very low toxic effects on these cells. Cinnamolide also caused remarkable changes in the morphology of the HeLa cancer cells and suppressed their colony forming potential. The AO/EB staining showed that this molecule inhibits the viability of cancer cells via induction of apoptotic cell death which was associated with increase in Bax and decrease in Bcl-2 levels. The apoptotic cells increased from 3.5% in control to around 59% in HeLa cells at 50 microM concentration. Cinnamolide treatment also led to activation of caspase-3 and caspase-9. It was also seen that Cinnamolide treatment led to a significant and dose-dependent loss of MMP in HeLa cancer cells. It also significantly inhibited the Akt/beta-catenin signalling pathway by reducing the levels of phosphorylated Akt and GSK-3beta. The results also showed that Cinnamolide suppressed the tumor volume and the tumor weight of the xenografted tumors. CONCLUSION: The results of this study indicate that Cinnamolide natural product has the potential to be developed as a promising anticancer agent against human cervical carcinoma.
Insecticidal and Antifeedant Activities of Malagasy Medicinal Plant (Cinnamosma sp.) Extracts and Drimane-Type Sesquiterpenes against Aedes aegypti Mosquitoes.[Pubmed:31731570]
Insects. 2019 Oct 25;10(11). pii: insects10110373.
The overuse of insecticides with limited modes of action has led to resistance in mosquito vectors. Thus, insecticides with novel modes of action are needed. Secondary metabolites in Madagascan plants of the genus Cinnamosma (Canellaceae) are commonly used in traditional remedies and known to elicit antifeedant and toxic effects in insect pests. Here we test the hypothesis that extracts of Cinnamosma sp. enriched in drimane sesquiterpenes are toxic and/or antifeedant to the yellow fever mosquito Aedes aegypti. We show that the bark and root extracts, which contain a higher abundance of drimane sesquiterpenes compared to leaves, were the most efficacious. Screening isolated compounds revealed cinnamodial to be the primary driver of adulticidal activity, whereas cinnamodial, polygodial, cinnafragrin A, and capsicodendrin contributed to the larvicidal activity. Moreover, an abundant lactone (cinnamosmolide) in the root extract synergized the larvicidal effects of cinnamodial. The antifeedant activity of the extracts was primarily contributed to cinnamodial, polygodial, and Cinnamolide. Parallel experiments with warburganal isolated from Warburgia ugandensis (Canellaceae) revealed that aldehydes are critical for-and a hydroxyl modulates-insecticidal activity. Our results indicate that plant drimane sesquiterpenes provide valuable chemical platforms for developing insecticides and repellents to control mosquito vectors.
Asymmetric Total Syntheses of Insulicolide A, 14- O-Acetylinsulicolide A, 6beta,9alpha-Dihydroxy-14- p-nitrobenzoylcinnamolide, and 7alpha,14-Dihydroxy-6beta- p-nitrobenzoylconfertifolin.[Pubmed:29957964]
Org Lett. 2018 Jul 20;20(14):4298-4301.
Asymmetric total syntheses of insulicolide A, 14- O-acetylinsulicolide A, 6beta,9alpha-dihydroxy-14- p-nitrobenzoyl Cinnamolide, and 7alpha,14-dihydroxy-6beta- p-nitrobenzoylconfertifolin have been achieved for the first time. The key steps in the synthesis include: (1) an iridium-catalyzed enantioselective polyene cyclization to construct the drimane core bearing two all-carbon quaternary chiral centers at C4 and C10 and (2) a cascade ozonolysis of the phenol ring to form the lactone fragment of the target molecules.
Drimane Sesquiterpenoids Noncompetitively Inhibit Human alpha4beta2 Nicotinic Acetylcholine Receptors with Higher Potency Compared to Human alpha3beta4 and alpha7 Subtypes.[Pubmed:29634269]
J Nat Prod. 2018 Apr 27;81(4):811-817.
The drimane sesquiterpenoids drimenin, Cinnamolide, dendocarbin A, and polygodial were purified from the Canelo tree ( Drimys winteri) and chemically characterized by spectroscopic methods. The pharmacological activity of these natural compounds were determined on halpha4beta2, halpha3beta4, and halpha7 nicotinic acetylcholine receptors (AChRs) by Ca(2+) influx measurements. The results established that drimane sesquiterpenoids inhibit AChRs with the following selectivity: halpha4beta2 > halpha3beta4 > halpha7. In the case of halpha4beta2 AChRs, the following potency rank order was determined (IC50's in muM): drimenin (0.97 +/- 0.35) > Cinnamolide (1.57 +/- 0.36) > polygodial (62.5 +/- 19.9) >> dendocarbin A (no activity). To determine putative structural features underlying the differences in inhibitory potency at halpha4beta2 AChRs, additional structure-activity relationship and molecular docking experiments were performed. The Ca(2+) influx and structural results supported a noncompetitive mechanism of inhibition, where drimenin interacted with luminal and nonluminal (TMD-beta2 intrasubunit) sites. The structure-activity relationship results, i.e., the lower the ligand polarity, the higher the inhibitory potency, supported the nonluminal interaction. Ligand binding to both sites might inhibit the halpha4beta2 AChR by a cooperative mechanism, as shown experimentally ( nH > 1). Drimenin could be used as a molecular scaffold for the development of more potent inhibitors with higher selectivity for the halpha4beta2 AChR.
Bioactive drimane sesquiterpenoids and aromatic glycosides from Cinnamosma fragrans.[Pubmed:28274626]
Bioorg Med Chem Lett. 2017 Apr 15;27(8):1754-1759.
Phytochemical investigation of the ethyl acetate and methanol extracts of the bark of Madagascan endemic and medicinal plant Cinnamosma fragrans led to the isolation of two drimane sesquiterpene derivatives: cinnafragroside A (1) and cinnafragrin E (2), two aromatic glycosides: 3,4,5-trimethoxyphenol 1-O-beta-d-apiofuranosyl-(1-->6)-beta-d-glucopyranoside (3) and 3,4-dimethoxyphenyl-1-O-beta-d-apiofuranosyl-(1-->6)-beta-d-glucopyranoside (4), together with 12 known compounds identified as: helicide (6), 1-(alpha-l-rhamnosyl(1-->6)-beta-d-glucopyranosyloxy)-3,4,5-trimethoxybenzene (7), vanilloloside (8), cinnamadin (9), ugandensolide (10), cinnamosmolide (11), Cinnamolide (12), polygodial (13), cinnamodial (14), bemadienolide (15), 4-isopropyl-6-methyl-alpha-tetralone (16), and capsicodendrin (17). Another new compound, 11-norcinnafragrolide-9-one (5), was obtained during chemical derivatization of capsicodendrin and gave a hint to understanding the structure required for the antiproliferative activity of 17. The structures of the new compounds were elucidated based on the interpretation of their spectroscopic data including one and two dimensional nuclear magnetic resonance (1D- and 2D-NMR) and mass spectroscopic data. All isolated compounds were evaluated against the hormone dependent breast cancer cell line MCF-7. Compound 17 exhibited the most potent activity with an IC50 value of 0.6muM. Our preliminary SAR study showed that the hydroxyl group at C-12' and the presence of conjugated carbonyl contribute to the antiproliferative activity.
Identification of a drimenol synthase and drimenol oxidase from Persicaria hydropiper, involved in the biosynthesis of insect deterrent drimanes.[Pubmed:28258968]
Plant J. 2017 Jun;90(6):1052-1063.
The sesquiterpenoid polygodial, which belongs to the drimane family, has been shown to be an antifeedant for a number of herbivorous insects. It is presumed to be synthesized from farnesyl diphosphate via drimenol, subsequent C-12 hydroxylation and further oxidations at both C-11 and C-12 to form a dialdehyde. Here, we have identified a drimenol synthase (PhDS) and a cytochrome P450 drimenol oxidase (PhDOX1) from Persicaria hydropiper. Expression of PhDS in yeast and plants resulted in production of drimenol alone. Co-expression of PhDS with PhDOX1 in yeast yielded drimendiol, the 12-hydroxylation product of drimenol, as a major product, and Cinnamolide. When PhDS and PhDOX1 were transiently expressed by agro-infiltration in Nicotiana benthamiana leaves, drimenol was almost completely converted into Cinnamolide and several additional drimenol derivatives were observed. In vitro assays showed that PhDOX1 only catalyses the conversion from drimenol to drimendiol, and not the further oxidation into an aldehyde. In yeast and heterologous plant hosts, the C-12 position of drimendiol is therefore likely to be further oxidized by endogenous enzymes into an aldehyde and subsequently converted to Cinnamolide, presumably by spontaneous hemiacetal formation with the C-11 hydroxyl group followed by oxidation. Purified Cinnamolide was confirmed by NMR and shown to be deterrent with an effective deterrent dose (ED50 ) of about 200-400 mug g(-1) fresh weight against both whiteflies and aphids. The putative additional physiological and biochemical requirements for polygodial biosynthesis and stable storage in plant tissues are discussed.
Antioxidant and alpha-amylase inhibitory activities of extract and isolates from Zygogynum pancheri subsp. arrhantum.[Pubmed:25034255]
J Asian Nat Prod Res. 2014 Dec;16(12):1132-8.
One new sesquiterpenoid (5R(*),8R(*),9R(*),10R(*))-Cinnamolide (8), and seven known compounds, 5-hydroxy-7-methoxyflavonone (1), 8-hydroxy-3-(4'-hydroxyphenyl)-6,7-(2'',2''-dimethylchromene)-tetralone (2), 8-hydroxy-3-(3',4'-dihydroxyphenyl)-6,7-(2'',2''-dimethylchromene)-tetralone (3), 1beta-E-O-p-methoxycinnamoyl-bemadienolide (4), 1beta-O-(E-cinnamoyl)-6alpha-hydroxy-9-epi-polygodial (5), 1beta-O-(E-cinnamoyl)-6alpha-hydroxypolygodial (6), and 1beta-O-E-cinnamoylpolygodial (7) were isolated from the ethyl acetate extract of barks of Zygogynum pancheri subsp. arrhantum (Winteraceae). The structures of these molecules were assigned predominantly based on spectral data. The structure of compound 8 was confirmed by X-ray crystallographic analysis. Compounds 2 and 3 exhibited significant antioxidant activity, whereas compounds 1 and 4-7 showed significant alpha-amylase inhibitory activity.
Growth inhibition of human colon carcinoma cells by sesquiterpenoids and tetralones of Zygogynum calothyrsum.[Pubmed:23517126]
J Nat Prod. 2013 Apr 26;76(4):710-4.
Bioassay-guided phytochemical investigation of Zygogynum calothyrsum using the human colon carcinoma cell lines COLO205 and KM12 led to the isolation of three new drimane-type sesquiterpenoids, 1beta-p-hydroxy-E-cinnamoyldrimeninol (1), 1beta-p-hydroxy-E-cinnamoyl-5alpha-hydroxydrimeninol (2), and methyl ether of 1beta-p-hydroxy-E-cinnamoyl-12alpha-methoxydrimeninol (3). Also isolated was the known 1beta-p-coumaroyloxypolygodial (4) together with two new tetralones, 3'-deoxyisozygolone A (5) and calothyrlone A (9), three known tetralones, isozygolone A (6), zygolone A (7), and 4'-O-methylzygolone A (8), and a known Cinnamolide (10). Compounds 1, 7, and 8 demonstrated higher cytotoxicity against COLO205 (GI50 18, 17, and 11 muM, respectively) and KM12 (GI50 14, 14, and 17 muM, respectively) than the other compounds.
Cinnamacrins A-C, cinnafragrin D, and cytostatic metabolites with alpha-glucosidase inhibitory activity from Cinnamosma macrocarpa.[Pubmed:17286431]
J Nat Prod. 2007 Feb;70(2):277-82.
Two new monomeric and two new dimeric drimane sesquiterpenes, cinnamacrins A-C (1-3) and cinnafragrin D (4), along with bemadienolide (5), capsicodendrin (6), cinnamodial (7), Cinnamolide (8), isopolygodial (9), and delta-tocotrienol (10), were isolated from Cinnamosma macrocarpa. The structures of the new compounds were determined by physical, chemical, and spectroscopic evidence. Capsicodendrin (6) and/or cinnamodial (7) are the major compounds in C. fragrans and C. macrocarpa, which are both widely used in Malagasy traditional medicine. The cytostatic activity as well as alpha-glucosidase inhibition and antiviral activities of the major constituents 6 and 7 and the compounds previously isolated from C. fragrans were evaluated.
Sesquiterpenes from Warburgia ugandensis and their antimycobacterial activity.[Pubmed:16153670]
Phytochemistry. 2005 Oct;66(19):2309-15.
The dichloromethane extract of the stem bark of Warburgia ugandensis afforded three new coloratane sesquiterpenes, namely: 6alpha,9alpha-dihydroxy-4(13),7-coloratadien-11,12-dial (1), 4(13),7-coloratadien-12,11-olide (2), and 7beta-hydroxy-4(13),8-coloratadien-11,12-olide (3), together with nine known sesquiterpenes, i.e., Cinnamolide-3beta-acetate (4), muzigadial (5), muzigadiolide (6), 11alpha-hydroxymuzigadiolide (7), Cinnamolide (8), 7alpha-hydroxy-8-drimen-11,12-olide (9), ugandensolide (10), mukaadial (11), ugandensidial (12), and linoleic acid (13). Their structures were assigned on the basis of 1D and 2D-NMR spectroscopic and GC-MS analysis. The compounds were examined for their antimycobacterial activity against Mycobacterium aurum, M. fortuitum, M. phlei and M. smegmatis; and the active constituents showed MIC values ranged from 4 to 128 microg/ml compared to the antibiotic drugs ethambutol (MIC ranged from 0.5 to 8 microg/ml) and isoniazid (MIC ranged from 1 to 4 microg/ml).


