Cirenshenoside SCAS# 226572-11-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 226572-11-8 | SDF | Download SDF |
| PubChem ID | 133561692 | Appearance | Powder |
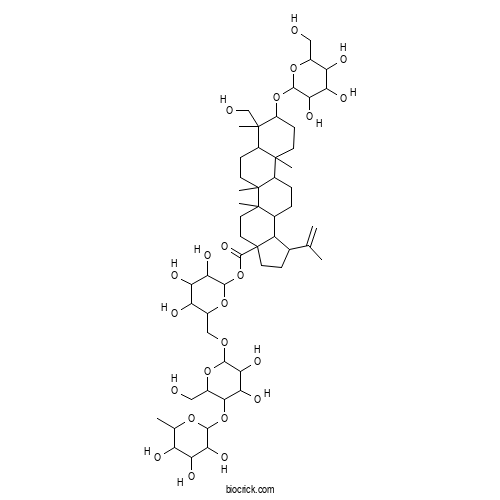
| Formula | C54H88O23 | M.Wt | 1105.3 |
| Type of Compound | Saponins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [6-[[3,4-dihydroxy-6-(hydroxymethyl)-5-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxymethyl]-3,4,5-trihydroxyoxan-2-yl] 8-(hydroxymethyl)-5a,5b,8,11a-tetramethyl-1-prop-1-en-2-yl-9-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-3a-carboxylate | ||
| SMILES | CC1C(C(C(C(O1)OC2C(OC(C(C2O)O)OCC3C(C(C(C(O3)OC(=O)C45CCC(C4C6CCC7C8(CCC(C(C8CCC7(C6(CC5)C)C)(C)CO)OC9C(C(C(C(O9)CO)O)O)O)C)C(=C)C)O)O)O)CO)O)O)O | ||
| Standard InChIKey | SAJIUZSFTKLJCY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C54H88O23/c1-22(2)24-10-15-54(49(69)77-48-42(67)38(63)35(60)28(74-48)20-70-45-43(68)39(64)44(27(19-56)73-45)76-46-40(65)36(61)33(58)23(3)71-46)17-16-52(6)25(32(24)54)8-9-30-50(4)13-12-31(51(5,21-57)29(50)11-14-53(30,52)7)75-47-41(66)37(62)34(59)26(18-55)72-47/h23-48,55-68H,1,8-21H2,2-7H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cirenshenoside S Dilution Calculator

Cirenshenoside S Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.9047 mL | 4.5237 mL | 9.0473 mL | 18.0946 mL | 22.6183 mL |
| 5 mM | 0.1809 mL | 0.9047 mL | 1.8095 mL | 3.6189 mL | 4.5237 mL |
| 10 mM | 0.0905 mL | 0.4524 mL | 0.9047 mL | 1.8095 mL | 2.2618 mL |
| 50 mM | 0.0181 mL | 0.0905 mL | 0.1809 mL | 0.3619 mL | 0.4524 mL |
| 100 mM | 0.009 mL | 0.0452 mL | 0.0905 mL | 0.1809 mL | 0.2262 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ginsenoyne E
Catalog No.:BCN0856
CAS No.:126146-63-2
- Notoginsenoside T1
Catalog No.:BCN0855
CAS No.:343962-53-8
- Ciwujianoside C3
Catalog No.:BCN0854
CAS No.:114906-74-0
- Ciwujianoside A1
Catalog No.:BCN0853
CAS No.:120768-65-2
- Siraitic acid A
Catalog No.:BCN0852
CAS No.:183374-15-4
- Siraitic acid B
Catalog No.:BCN0851
CAS No.:183374-16-5
- 11-Deoxymogroside IIIE
Catalog No.:BCN0850
CAS No.:1793003-47-0
- 11-Oxomogroside I
Catalog No.:BCN0849
CAS No.:918972-06-2
- Mogroside II-B
Catalog No.:BCN0848
CAS No.:942615-25-0
- Mogroside I-A1
Catalog No.:BCN0847
CAS No.:88901-46-6
- Mogroside I-E1
Catalog No.:BCN0846
CAS No.:88901-39-7
- Neomogroside
Catalog No.:BCN0845
CAS No.:189307-15-1
- Ginsenoside F11
Catalog No.:BCN0858
CAS No.:115038-42-1
- Ginsenoside Rh8
Catalog No.:BCN0859
CAS No.:343780-69-8
- Ginsenoside Ra3
Catalog No.:BCN0860
CAS No.:90985-77-6
- 1,3,5-tricaffeoylquinic acid
Catalog No.:BCN0861
CAS No.:1073897-80-9
- Heratomol
Catalog No.:BCN0862
CAS No.:61265-07-4
- Secologanin
Catalog No.:BCN0863
CAS No.:19351-63-4
- Clinoposaponin D
Catalog No.:BCN0864
CAS No.:1822328-43-7
- Clinoposaponin VIII
Catalog No.:BCN0865
CAS No.:152020-04-7
- Clinoposaponin X
Catalog No.:BCN0866
CAS No.:159122-00-6
- Clinoposaponin XI
Catalog No.:BCN0867
CAS No.:159122-01-7
- Clinoposaponin I
Catalog No.:BCN0868
CAS No.:152580-76-2
- Clinoposaponin IX
Catalog No.:BCN0869
CAS No.:159121-99-0
Qualitative and quantitative determination of nine main active constituents in Pulsatilla cernua by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry.[Pubmed:21268254]
J Sep Sci. 2011 Feb;34(3):308-16.
A novel qualitative and quantitative method using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) was developed for simultaneous determination of the nine major active constituents in Pulsatilla cernua (Thunb.) Bercht. et Opiz., namely anemoside A3 (1), anemoside B4 (2), 23-hydroxybetulinic acid (3), Cirenshenoside S (4), pulsatilloside B (5), pulsatilloside C (6), oleanolic acid (7), ajugasterone C (8) and beta-ecdysterone (9), respectively. A Sapphire C18 column (250 mm x 4.6 mm, 5 mum) and gradient elution were used during the analysis. The identification and quantification of the analytes were achieved on a hybrid quadrupole linear ion trap mass spectrometer. Multiple-reaction monitoring (MRM) scanning was employed for quantification with switching electrospray ion source polarity between positive and negative modes in a single run. All calibration curves showed good linearity (r(2) > 0.9948) within the test ranges. The intra and interday variations for nine analytes were less than 3.95 and 3.78%, respectively. The developed method was successfully applied to determine the investigated compounds in 15 batches of natural and cultured samples of P. cernua. The results indicated that the method was simple, rapid, specific and reliable, which is helpful to comprehensive evaluation of quality of P. cernua.
Structures of four new triterpenoid saponins from the leaves of Oplopanax elatus Nakai.[Pubmed:15338878]
Yao Xue Xue Bao. 2004 May;39(5):354-8.
AIM: Isolation and structural elucidation of the triterpenoid saponins of Oplopanax elatus Nakai. METHODS: Solvent extraction and column chromatography were used to isolate the triterpenoid saponins, physico-chemical constants and spectroscopic analysis were employed for structural elucidation. RESULTS: Four newtriterpenoid saponins named Cirenshenoside S (1), cirenshenoside T (2), cirenshenoside U (3) and cirenshenoside V (4) were isolated, and their structures were elucidated to be 3-O-beta-D-glucopyranosyl 3beta,23-dihydroxylup-20 (29)-en-28-oic acid 28-O-alpha-L-rhamnopyranosyl (1 --> 4)-beta-D-glucopyranosyl (1 --> 6)-beta-D-glucopyranoside (1), 3-O-beta-D-glucopyranosyl hederagenin 28-O-alpha-L-rhamnopyranosyl (1 --> 4)-beta-D-glucopyranosyl (1 --> 6)-beta-D-glucopyranoside (2), 3-O-beta-D-glucopyranosyl 3beta-hydroxyolean-9(11),12-dien-28-oic acid 28-O-alpha-L-rhamnopyranosyl (1 --> 4)-beta-D-glucopyranosyl (1 --> 6)-beta-D-glucopyranoside (3) and 3alpha-hydroxyolean-12-dien-23,28-dioic acid 28-O-alpha-L-rhamnopyranosyl (1 --> 4 )-beta-D-glucopyranosyl (1 --> 6)-beta-D-glucopyranoside (4), respectively. CONCLUSION: Compounds 1-4 are new triterpenoid saponins and isolated from the leaves of Oplopanax elatus Nakai for the first time.


