11-Oxomogroside ICAS# 918972-06-2 |
Quality Control & MSDS
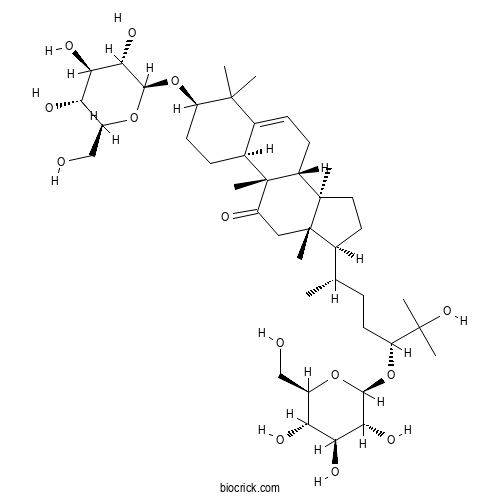
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 918972-06-2 | SDF | Download SDF |
| PubChem ID | 154831846 | Appearance | Powder |
| Formula | C42H70O14 | M.Wt | 799 |
| Type of Compound | Saponins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R,8R,9S,10S,13S,14R,17S)-17-[(2S,5R)-6-hydroxy-6-methyl-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyheptan-2-yl]-4,4,9,13,14-pentamethyl-3-[(2S,3S,4R,5R,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,2,3,7,8,10,12,15,16,17-decahydrocyclopenta[a]phenanthren-11-one | ||
| SMILES | CC(CCC(C(C)(C)O)OC1C(C(C(C(O1)CO)O)O)O)C2CCC3(C2(CC(=O)C4(C3CC=C5C4CCC(C5(C)C)OC6C(C(C(C(O6)CO)O)O)O)C)C)C | ||
| Standard InChIKey | TUFDSEMWRYIOBO-IKXRKSGISA-N | ||
| Standard InChI | InChI=1S/C42H70O14/c1-20(9-13-29(39(4,5)52)56-37-35(51)33(49)31(47)25(19-44)54-37)21-15-16-40(6)26-12-10-22-23(42(26,8)27(45)17-41(21,40)7)11-14-28(38(22,2)3)55-36-34(50)32(48)30(46)24(18-43)53-36/h10,20-21,23-26,28-37,43-44,46-52H,9,11-19H2,1-8H3/t20-,21-,23-,24-,25+,26+,28+,29+,30-,31+,32+,33-,34-,35+,36+,37-,40+,41-,42+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

11-Oxomogroside I Dilution Calculator

11-Oxomogroside I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2516 mL | 6.2578 mL | 12.5156 mL | 25.0313 mL | 31.2891 mL |
| 5 mM | 0.2503 mL | 1.2516 mL | 2.5031 mL | 5.0063 mL | 6.2578 mL |
| 10 mM | 0.1252 mL | 0.6258 mL | 1.2516 mL | 2.5031 mL | 3.1289 mL |
| 50 mM | 0.025 mL | 0.1252 mL | 0.2503 mL | 0.5006 mL | 0.6258 mL |
| 100 mM | 0.0125 mL | 0.0626 mL | 0.1252 mL | 0.2503 mL | 0.3129 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mogroside II-B
Catalog No.:BCN0848
CAS No.:942615-25-0
- Mogroside I-A1
Catalog No.:BCN0847
CAS No.:88901-46-6
- Mogroside I-E1
Catalog No.:BCN0846
CAS No.:88901-39-7
- Neomogroside
Catalog No.:BCN0845
CAS No.:189307-15-1
- 4-p-Menthan-1,8-diol
Catalog No.:BCN0844
CAS No.:565-48-0
- Silybin A
Catalog No.:BCN0843
CAS No.:36804-17-8
- Malabaricone A
Catalog No.:BCN0842
CAS No.:63335-23-9
- Baishouwubenzophenone
Catalog No.:BCN0841
CAS No.:115834-34-9
- Malabaricone C
Catalog No.:BCN0840
CAS No.:63335-25-1
- Isosecotanapartholide
Catalog No.:BCN0839
CAS No.:102926-01-2
- 8-Epiloganin
Catalog No.:BCN0838
CAS No.:79172-04-6
- Otobaphenol
Catalog No.:BCN0837
CAS No.:10240-16-1
- 11-Deoxymogroside IIIE
Catalog No.:BCN0850
CAS No.:1793003-47-0
- Siraitic acid B
Catalog No.:BCN0851
CAS No.:183374-16-5
- Siraitic acid A
Catalog No.:BCN0852
CAS No.:183374-15-4
- Ciwujianoside A1
Catalog No.:BCN0853
CAS No.:120768-65-2
- Ciwujianoside C3
Catalog No.:BCN0854
CAS No.:114906-74-0
- Notoginsenoside T1
Catalog No.:BCN0855
CAS No.:343962-53-8
- Ginsenoyne E
Catalog No.:BCN0856
CAS No.:126146-63-2
- Cirenshenoside S
Catalog No.:BCN0857
CAS No.:226572-11-8
- Ginsenoside F11
Catalog No.:BCN0858
CAS No.:115038-42-1
- Ginsenoside Rh8
Catalog No.:BCN0859
CAS No.:343780-69-8
- Ginsenoside Ra3
Catalog No.:BCN0860
CAS No.:90985-77-6
- 1,3,5-tricaffeoylquinic acid
Catalog No.:BCN0861
CAS No.:1073897-80-9
Two New Cucurbitane Glycosides from the Fruits of Siraitia grosvenori.[Pubmed:30982796]
Chem Pharm Bull (Tokyo). 2019 Jul 1;67(7):721-724.
Two novel cucurbitane glycosides, named as 11-Oxomogroside III A1 and 7beta-methoxy-mogroside V, along with sixteen known ones were isolated from the fruits of Siraitia grosvenori SWINGLE. The structures of the new compounds were characterized by chemical and extensive spectral methods.
[Simultaneous determination of six cucurbitane triterpene glycosides in Siraitia grosvenorii fruits using high performance liqid chromatography].[Pubmed:18959251]
Se Pu. 2008 Jul;26(4):504-8.
Siraitia grosvenorii, a traditional Chinese fruit, belongs to the family Cucurbitaceae and has been used as a pulmonary demulcent and emollient for the treatment of dry cough, sore throat, dire thirst, and constipation in folk medicine. A high performance liquid chromatographic method was developed for simultaneous determining the contents of mogroside V, mogroside IV A, mogroside III, 11-Oxomogroside III, mogroside II E and 11-Oxomogroside II E in Siraitia grosvenorii fruits. The chromatographic analysis was carried out on a ZORBAX SB-C18 column (150 mm x 4.6 mm, 5 microm). The mobile phase was water (A) and acetonitrile (B) with gradient elution (0-3 min, 20% B - 30% B; 3-8 min, 30% B - 35% B; 8-9 min, 35% B). The flow rate was maintained at 0. 8 mL/min. The detection wavelength was set at 203 nm and the column temperature was controlled at 30 degrees C. The sample injection volume was 10 microL. The calibration curves were linear over the ranges of 0.04 - 1.0 mg/mL, 0.011 - 0.68 mg/mL, 0.010 - 0.80 mg/mL, 0.0097 - 0.58 mg/mL, 0.025 - 1.0 mg/mL and 0.013 - 0.76 mg/mL (r > 0.999 1) for the above cucurbitane triterpene glycosides, respectively. The average recoveries were 99. 65% for mogroside V , 101.6% for mogroside IV A, 97. 05% for mogroside m, 103. 1% for 11-Oxomogroside I, 99. 25% for mogroside II E, and 103.0% for 11-Oxomogroside II E, with the relative standard deviations of 0.83%, 3.1%, 1.9%, 3.3%, 0.59% and 2.0%, respectively. This simple, rapid and accurate method is suitable for quality control and determination of raw materials and products of Siraitia grosvenorii fruits.
Cucurbitane glycosides from unripe fruits of Siraitia grosvenori.[Pubmed:17603208]
Chem Pharm Bull (Tokyo). 2007 Jul;55(7):1082-6.
Studies on the constituents of the unripe fruits of Siraitia grosvenori led to the isolation of three new cucurbitane triterpene glycosides, 11-Oxomogroside III (10), 11-dehydroxymogroside III (11), and 11-Oxomogroside IV A (12). Their structures were determined on the basis of detailed analyses of 1D, 2D-NMR spectroscopic methods. All of the compounds isolated from the unripe fruits of S. grosvenori were tested for cytotoxic activities against tumor cells, HCT-116 and SMMC-7721.
Cucurbitane glycosides from the fruits of Siraitia gros venorii and their inhibitory effects on Epstein-Barr virus activation.[Pubmed:17477572]
J Nat Prod. 2007 May;70(5):783-8.
Six new cucurbitane glycosides, mogroside II B (2), 11-deoxymogroside III (4), 7-oxomogroside II E (5), 7-oxomogroside V (6), 11-Oxomogroside II A1 (7), and 11-Oxomogroside IV A (8), and two known but new naturally occurring cucurbitane glycosides, mogroside II A1 (1) and mogroside III A2 (3), were isolated from an ethanol extract of the fruits of Siraitia grosvenorii. Upon evaluation of compounds 1-8 for inhibitory effects against the Epstein-Barr virus early antigen (EBV-EA) activation induced by 12-O-tetradecanoylphorbol-13-acetate (TPA), all compounds exhibited inhibitory effects with IC50 values of 346-400 mol ratio/32 pmol TPA. In addition, compounds 1-8 showed weak inhibitory effects on activation of (+/-)-(E)-methyl-2-[(E)-hydroxyimino]-5-nitro-6-methoxy-3-hexemide (NOR 1), a nitric oxide (NO) donor.
Cucurbitane glycosides from unripe fruits of Lo Han Kuo (Siraitia grosvenori).[Pubmed:17015982]
Chem Pharm Bull (Tokyo). 2006 Oct;54(10):1425-8.
From the unripe fruits of Lo Han Kuo (Siraitia grosvenori), a Chinese medicinal plant, two new cucurbitane triterpene glycosides, 20-hydroxy-11-Oxomogroside IA(1) (1) and 11-Oxomogroside IIE (2), were isolated along with five known cucurbitane glycosides, 11-Oxomogroside IA(1) (3), mogroside IIE (4), mogroside III (5), mogroside IVA (6), and mogroside V (7), and two flavonoid glycosides, kaempferol 7-O-alpha-L-rhamnopyranoside (8) and kaempferol 3,7-di-O-alpha-L-rhamnopyranoside (9). Their structures were determined on the basis of detailed analyses of 1D, 2D-NMR spectroscopic methods and by comparing with literature values. This paper describes the first investigation of unripe bitter Lo Han Kuo fruits.


